Before we begin, here’s a bit of Ruby’s background to give you some context:
Ruby has been a science communicator and scientific partnership liaison for more than 20 years. She has had extensive research and project management experience at various academic institutions with roles that develop and expedite business development strategies involving multi-disciplined key stakeholders.
She has led and published (many are featured in media) over 60+ highly cited work (>5590 times with an H-index of 35). She is a recognised expert in the discipline of transcriptomics, integrative bioinformatics, molecular genetics, therapeutic targets and clinical trials (specifically in cardiovascular diseases, cancer and metabolic syndrome). She was a named NHMRC Peter Doherty fellow (2005-8) and a UNSW Global Postdoctoral fellow (2009-14). She has won over A$6.4 million in competitive research funding (Category 1) from NHMRC project grants, ARC LIEF, MRFF as well as NSW Cancer Infrastructure grants and philanthropic grants. Specifically in the last few years, she has secured research funding in the disciplines of genomics, cardiovascular diseases, obesity, cancer biology, tissue/pathogen/phage biobanking as well as phage therapy.
During her time as the president of AGTA (2013-5), she continued to implement co-convenors for its annual meeting with a quiet room for delegates with carer duties, ECR-MCR pairing to chair sessions and 50:50 gender balanced international and local invites. AGTA proudly continues this equity diversity and inclusion guidelines in its conferences, workshops and symposiums. She is part of UNSW Women in Research and advocates for girls and women in STEMM and does pro bono career coaching.
Over last 4.8 years, led by Jon Iredell, Australian clinicians, scientists and regulators have been working collectively to implement bacteriophage therapy in Australia. This work has culminated in the creation of “Phage Australia”, and its AusPhageNet Clinical and AusPhageNet Bio network. As we seek additional funding from MRFF Frontiers Stage 2, philanthropy and venture capital to establish an Australia-wide resource, here are some of my reflections.
I have previously outlined the importance of balanced co-investment from public and private sectors in a peer-reviewed biobanking discussion paper [1]. In the last few years, we have linked phage characterisation to existing publicly funded bacterial pathogen surveillance systems and the standardised monitoring of phage therapy in public hospitals here in Australia.
Now, to ensure sustainability, we must define commercialisation pathways. The biobanking arm of Phage Australia must embrace novel regulatory pathways, as yet undefined reimbursement mechanisms, supply chain and manufacturing/distribution/logistics and completely new clinical services.
We commissioned Deloitte for a market sounding analysis, funded by NSW Health. This revealed that risk-averse investors will likely be unwilling to support investment at this stage. Thus, over the last 12 months (MRFF Frontier Stage 1 funded), we were able to crystalise ideas and address the main issues, which I will outline here.
1. Robust manufacturing and distribution (including supply chain and biobanking).
We collaborate with UNSW Recombinant Products Facility (Chris Marquis, Helene Lebhar, Ali Khalid) for phage purification processes (see Jessica Sacher’s articles [2]), Citizen Phage Library (Ben Temperton) and Phage Australia network for potential phage supplies, and our own testing on distribution/stability testing of Pae7 phage (see Stephanie Lynch’s article [3].) We have already produced and distributed a batch of clean home-grown phages to all our partners for testing. I am acutely aware of the effort and investment needed for a biomanufacturing and bioprocessing plant dedicated to phage.
We have demonstrated strong feasibility of networked biobanking at state level (NSW Govt funding for Pathogen [4] and Phage [5] biobanks, WA health department endorsed implementation task force); national level and international level, i.e., Israel Phage Biobank (Ronen Hazan, Ran Nir-Paz), LysenTech (South Korea, Heejoon Myung), Denmark (Lars Hansen, Nikoline Olsen), Belgium (Jean-Paul Pirnay, Pieter-Jan Ceyssens, Rob Lavigne) etc.
In the last 12 months we have demonstrated operational logistics in terms of phage exchange at state, national and international level, standardised workflows for sample submission and patient screening, genomics sequencing, patient isolate and phage screening under STAMP [6] and standardised data curation (see Jan Zheng’s articles [7]) to produce Australian Phage Passport (to be published soon).
It is important to note that the availability of diverse high-quality phages is facilitated by our bioprospecting programs (Stephen Stick [8], Anthony Kicic, Trevor Lithgow, Jeremy Barr) and our national and international linkages. The accurate representation of bacterial strains that is our stock-in-trade enables us to identify which phages we need on the shelves and which phages can be lower volumes, as well as which ones can be available in a few days or a week. This sets us apart from all of our competitors and is the power of our national network.
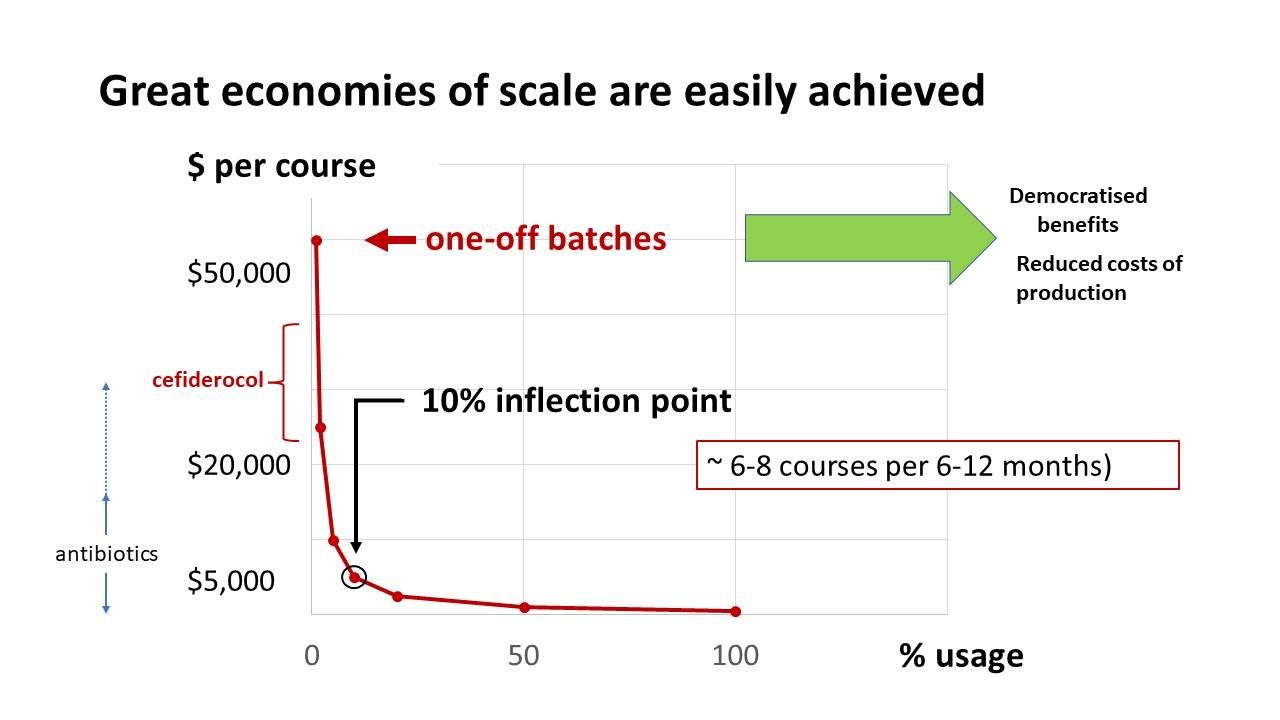
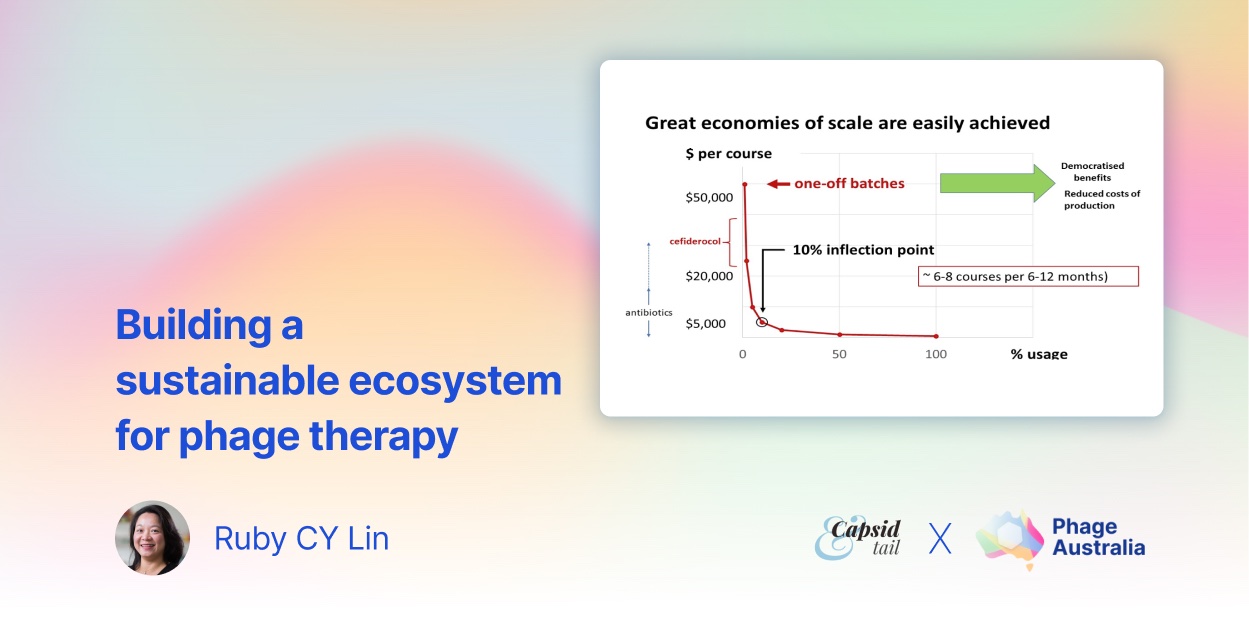
Fig. 1. Economies of scale for phage production can be easily achieved. (credit: Jon Iredell).
Here, we present our calculations on phage production [9]. Phage production is a growth process. We have reconciled with the fact that a small batch costs almost as much as a large batch, and efficiencies of scale are very important. For a ~$60,000 AUD GMP-grade one off batch of ~1,000 doses (~30 courses) means we can produce phages for about the cost of a modestly priced antibiotics. Accounting for a clinical trial with 50% wastage, this is well beyond the inflection point for economies of scale.
2. Effective regulatory pathways.
Facilitated by NSW Health (Tony Penna, Laura Collie, Julia Warning and Sian Hope), we are working with representatives from TGA (Therapeutic Goods Administration) and OGTR (Office of Gene Technology Regulator) to regulate phage therapy in Australia. We will share this in due course.
We have established a clinical network and a nationally approved protocol to regulate phage therapy under the clinical trial framework i.e., Safety and tolerability of Standardised Treatment and Monitoring of Phage therapy (STAMP) in adults and children (Australia and New Zealand Clinical Trial Registry: ACTRN12621001526864 [10]). This is endorsed by ASID (Australasian Society for Infectious Diseases), ASID paediatric infectious diseases arm (ANZPID), CFA (Cystic Fibrosis Australia) and TSANZ (Thoracic Society of Australia and New Zealand).
Standardised treatment protocols endorsed by expert groups together with clinicians and nurses, training & education are an integral part of Phage Australia’s value proposition. To note, this will be developed further towards “Phage Therapy for Critical Infection”, similar to the S100-style training i.e., Critical Care Short Courses at both Westmead and the Alfred that have been self- sustainable for over a decade. Furthermore, we have designed a Phage Therapy Education Package for scientists and consumers (Sian Hope, Ruby Lin) that is waiting to be signed off. I will detail the importance of industry participation in training workforce, specifically PhD students [11], in the next instalment.
3. A roadmap for clinical Pathways and the patient journey.
In collaboration with NSW Health (Sian Hope, Julia Warning, Laura Collie), we mapped out the patient journey for phage therapy in comparison to patients being treated with antibiotics only at a public hospital, e.g., Westmead Hospital. To note, Jon Iredell and Ameneh Khatami remain the only two clinicians in Australia with phage therapy and therapeutic monitoring experience (see our publications on Phage Australia website), as well as the process of phage-pathogen matching towards production of a purified phage for intravenous administration.
A health implementation plan for phage therapy in the clinic (Manisha Yapa) will ensure that the most efficient implementation of this new therapy is understood well in advance of the rollout once it is ‘main streamed’.
Health economics are outlined below, and phage production cost is outlined above.
4. Cost-effective payment and reimbursement pathways.
We commissioned HTAnalysts [12] to map out in the first instance feasibility and impact of phage therapy for AMR (antimicrobial resistance) sepsis [13] and prosthetic joint infection (PJI).
Substantial savings are made with phage therapy, i.e., $122.8M over a 6-year window for PJI and $67M for sepsis (over 3 years). See link to the reports. Briefly the findings are outlined here.
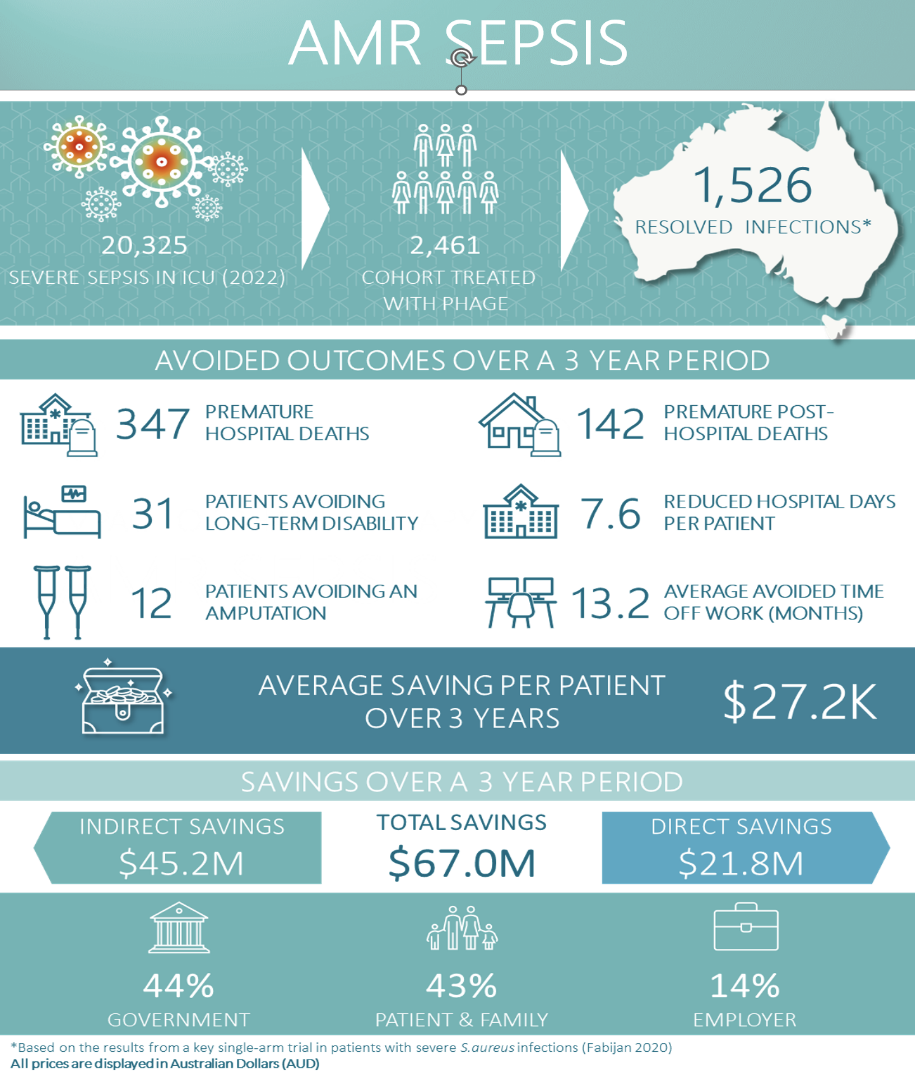
Fig. 2. Summarised figure of savings by introducing phage therapy as part of AMR sepsis clinical service. Refer to HTAnalyst report.
The key findings for AMR sepsis are (as outlined in the report [AMR Sepsis PDF]):
- If all patients across Australia with AMR sepsis (2,461 patients) were treated with bacteriophage therapy, the total savings over 3 years were estimated to be $67.0 million corresponding to an average saving of $27,239 per patient.
- If all patients across NSW with AMR sepsis (782 patients) were treated with bacteriophage therapy, the total savings over 3 years were estimated to be $21.3 million.
- The total direct savings (healthcare related savings) were $21.8 million, all of which was paid for by the government.
- The total indirect savings (non-healthcare related savings) were $45.2 million, most of which was paid for by patients (63%) followed by employers (20%) and the government (17%).
- Length of stay for patients with AMR sepsis is estimated to be reduced by an average of 7.6 days per patient.
- Resolved infection from bacteriophage is estimated to reduce average time off work by 13.2 months per patient, reducing potential lost income for patients who were previously working prior to their hospital admission.
- Over the 3-year time horizon, 31 patients with an AMR sepsis infection would avoid long term disability (classified as not returning to work within 3 years of discharge), resulting in the reduction of home care, disability support, and the need for a primary caregiver.
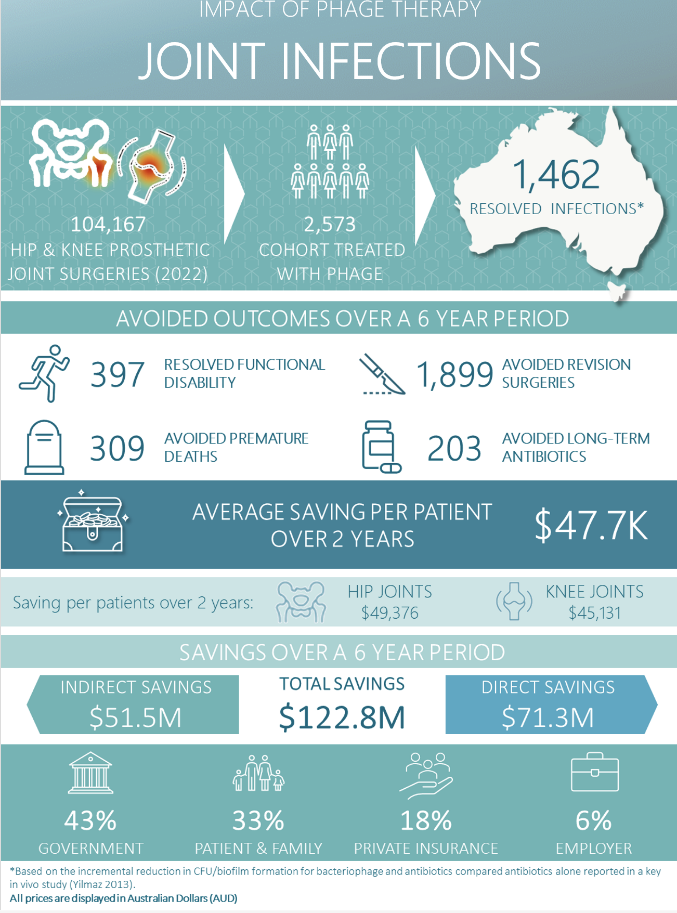
Fig. 3. Summarised figure of savings by introducing phage therapy as part of prosthetic joint infections clinical service. Refer to HTAnalyst report.
The key findings for PJI are:
- If all patients across Australia with prosthetic knee and hip infections (2,573 patients) were treated with bacteriophage therapy, the total savings over 6 years were estimated to be $122.8 million in 2022 corresponding to an average saving of $47,736 per patient over 2 years [^pji-pdf].
- If all patients across NSW with prosthetic knee and hip infections (818 patients) were treated with bacteriophage therapy, the total savings over 6 years were estimated to be $38.8 million in 2022 corresponding to an average saving of $47,424 per patient over 2 years [^pji-pdf].
- The total direct savings (healthcare relating savings) were $71.3 million most of which was attributed to the government (63%) and private health insurers (33%). Savings were also partially attributed to patients and their families (4%).
- The total indirect savings (non-health care related savings) were $51.5 million most of which was attributed to patients and their families (67%), followed by the government (19%) and employers (14%).
- The highest saving per patients were reported for hip joints ($49,376) and patients with chronic infections ($45,131). Greater saving per patients were incurred in the first 4 years ($50,846 for year 0-2, $46,153 for year 2-4 and $25,207 for year 4-6).
These analyses will be repeated using real data in 3 years’ time and we expect this will be the first real-world cost-effectiveness data available for phage therapy.
Based on these health economics calculations, we can state that phage therapy is an efficient spend of public health dollars right now.
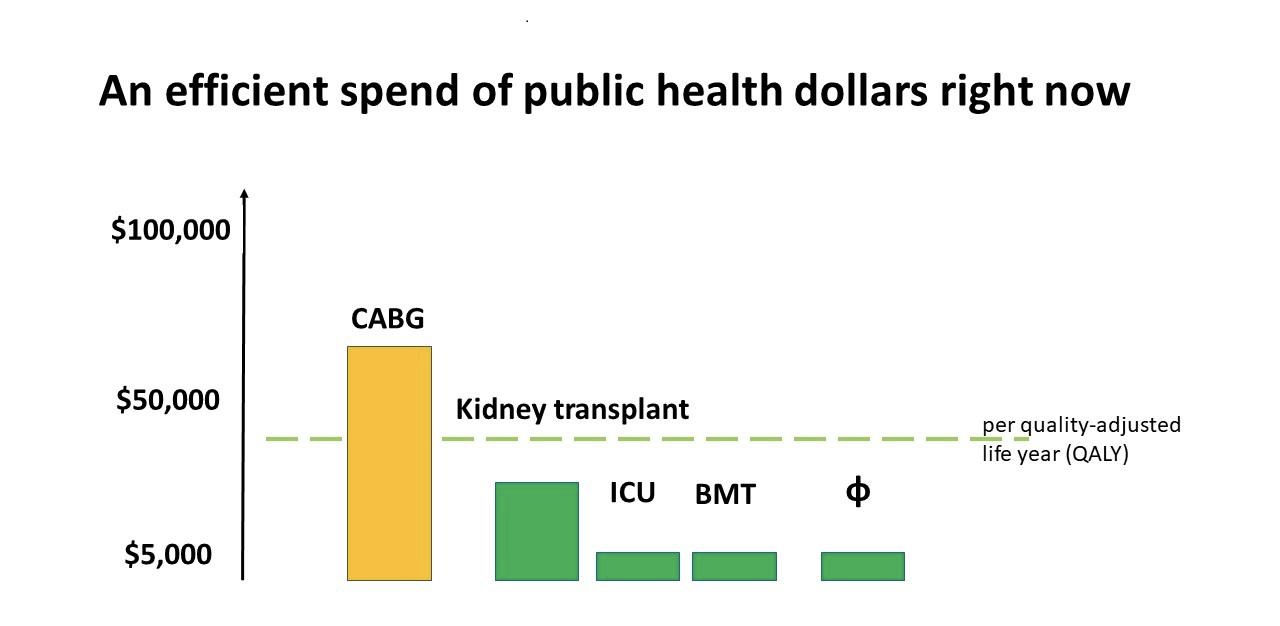
Fig. 4. Comparison of cost of procedures spent by current public health dollars in Australia. (credit: Jon Iredell).
Jon Iredell reviewed the decisions by Australian government (PBS, Pharmaceutical Benefit Scheme [14]) which indicated a willingness to pay $15,000 – $45,000 AUD for a quality adjusted life year (the quality adjusted life year is a health economist measure of return on health investment). This graph shows that ICU and bone marrow transplant services are very good value, as are kidney transplants. Coronary bypass grafting (CABG) is over $50,000 AUD. Our externally commissioned modelling showed that phage therapy to be at least as good value as bone marrow transplantation and ICU services. Importantly, this is a lot cheaper to implement.
Aligning reimbursement [15] and regulatory policies for phage therapy is our challenge (more on this in my next blog) and phage therapy must remain under the watchful eye of research and drug safety committees for now. The Australian Antimicrobial Resistance Network – AAMRNet – has set out a position statement [16] around funding of novel antimicrobials in Australia but this remains an open conversation.
At Phage Australia, we have scheduled for Health Technology Assessment and Market Access Analysis on Phage Therapy, in order to inform PBAC and MSAC.
A recent example of experimental therapy being accepted into all aspect of healthcare system is Novartis’ outcome-based healthcare strategy of introducing CAR-T cell therapy into the mainstream market [17] based on one single-arm data in adults and paediatric patients.
Part Two
In the next part of Ruby’s analysis, Ruby will dive deeper into what target populations phage therapy should focus on, and discuss some of potential revenue streams and business models. Stay tuned!
Feel like skipping ahead and reading the whole white paper? Read it on Phage Australia
Downloads
- AMR Sepsis PDF: Impact of Phage Therapy on AMR Sepsis, prepared by HTANALYSTS https://dl.phage.directory/WIMR01_AMRSepsis_WP_FINAL_26July2022.pdf
- PJI PDF: Impact of Phage Therapy on Joint Infections, prepared by HTANALYSTS https://dl.phage.directory/WIMR01_PJI_WP_FINAL_26July2022.pdf
References
- Phage Biobank: Present Challenges and Future Perspectives. RCY Lin, JC Sacher, PJ Ceyssens, J Zheng, A Khalid, JR Iredell & The Australian Phage Network. Current Opinions in Biotechnology https://doi.org/10.1016/j.copbio.2020.12.018
- https://www.phageaustralia.org/blog/phage-batch-results, https://phage.directory/capsid/phage-making-first-results
- https://phage.directory/capsid/phage-shipping-around-australia
- NSW Pathogen Biobank, Jon Iredell, Ruby Lin, Tania Sorrell, Dominic Dwyer, Wieland Meyer, Sharon Chen et al. $100k awarded, 2019-2023.
- NSW Phage Biobank, Jon Iredell, Ruby Lin. $100k awarded, 2020-4
- https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=382817&isReview=true
- https://phage.directory/capsid/how-to-organize-phage-biobank-data; https://phage.directory/capsid/how-to-name-a-phage; https://phage.directory/capsid/how-to-track-phages
- “On country” bioprospecting, phage naming in language, rapid through-put susceptibility testing; Ng RN, Grey LJ, Vaitekenas A, McLean SA, Rudrum JD, Laucirica DR, et al. Development and validation of a miniaturized bacteriophage host range screening assay against antibiotic resistant Pseudomonas aeruginosa. J Microbiol Methods. 2021;190:106346.
- Based on quotes from third party GMP-phage production and hospital statistics.
- https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=382817&isReview=true
- https://www.science.org/content/article/controversial-plan-brings-ph-d-students-biotech-training
- https://htanalysts.com.au/
- This included data from our phage therapy clinical trial: Petrovic Fabijan, A., Lin, R.C.Y., Ho, J. et al. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol 5, 465–472 (2020). https://doi.org/10.1038/s41564-019-0634-z
- https://www.pbs.gov.au/browse/publications
- https://hbr.org/2019/10/making-life-saving-medical-treatments-more-affordable
- https://www.mtpconnect.org.au/images/AAMRNet_PositionStatement_PricingReimbursement_2022.pdf
- https://www.novartis.com/research-development/technology-platforms/cell-therapy/car-t-cell-therapy-and-beyond