Welcome back to the third installment of the Sustainable Phage Therapy series by Ruby Lin!
In Part One: Phage manufacturing, regulations, health economics & reimbursement pathways, Ruby discusses the need for a sustainable ecosystem for phage therapy, which includes co-investment from the public and private sectors, robust manufacturing and distribution, and effective regulatory pathways. The blog also discusses the potential benefits of phage therapy, including cost savings and improved patient outcomes.
In Part Two, Ruby explores pathways to generating revenue through clinical services, biobanking, and companion diagnostics, and phage products.
In Part Three, we wrap the series with a discussion of data-driven business models and concludes the series with thoughts on a development pathway and timeline for a sustainable ecosystem for phage therapy, and the challenges that need to be addressed to make phage therapy a success.
6. Broad potential revenue streams.
A brief overview of the other income streams from Part Two:
- Income stream 1: Clinical Service
- Income Stream 2: Cost-recovery for biobanking services
- Income stream 3: Packaged phage and bacterial products
- Income stream 4: Synthetic products
- Income stream 5: Personalised products
Income Stream 6: DATA
Data and product provenance is central issues in biology. As the field moves towards digitalisation [1] we identified [2] several enabling features such as centralised governance, standardised protocols, standardised definition (e.g., Data Dictionary used in REDcap to capture STAMP trial data) and good data management that are needed to facilitate contribution of data (clinical and lab data associated with a specific phage) and product (phage and/or bacteria, see Australia Phage Passport) to the Phage Australia biobank and data system.
An integrated platform combining Artificial Intelligence and Distributed Ledger Technology [3] and internationally standardised quality management system (e.g., ISO9001 accreditation) can ensure seamless audit and quality assurance. This in turn boosts confidence amongst users, stakeholders and investors for a specific product. We are in the process of working out a ledger system for phage product and its companion data to address sample and data provenance. To make it fun, cryptocurrency, e.g., Phage Coin has been discussed but we proceed with caution [4].
The data management system and integrated platform we are building at Phage Australia is likely to be attractive to investors. A striking example of success with data utilisation and data linkage is Tempus [5] - a Chicago-based technology company that offers genetic testing and aggregates clinical information for cancer [6]. Groupon’s initial investment in 2015 enabled Tempus to be valued at $8.1 billion USD after raising $200 million in equity financing and $250 million in convertible debt by December 2020. Tempus said at the time that their investors included Baillie Gifford, Google, Franklin Templeton, Novo Holdings and funds managed by T. Rowe Price Group Inc. Currently Tempus is a company valued at $3.1 billion USD and its database enables clinicians to diagnose specific cancer type (and the best specific therapy to match) in real time. There is no such venture in the infectious diseases space. Phage Australia’s effort can fill this gap.
There are also many examples of co-investment from public and private sectors [7] to bring different streams of income into an entity by combining a physical collection of samples (e.g., a biobank) integrated with an online collection of data (decentralised databases). Sweden’s national collaboration for genomic medicine [8] (from sequencing to clinical practice) and Scandinavia’s effort in combining public and private effort to utilise data to innovate [9] healthcare are good examples.
Funding from MRFF Frontiers Stage 2, philanthropy and investors can expedite this whole process.
Income Stream 7: GENOMIC SEQUENCING
For the duration of MRFF Frontiers Stage 1, Phage Australia was fortunate to have the support of several industry partners in DNA sequencing to serve the projects within the Phage Australia network.
By partnering with AGRF and the Westmead Institute Core Facility for Genomics, we were able to validate the whole genome sequencing pipeline and turn it into a self-sustainable operation. For example, a cost-recovery model was implemented in the first instance to recoup the cost of labour and reagents from a discounted rate to Phage Australia network users for phage, bacteria and metagenome sequencing. All negotiated discounts from Stage 1 were passed on to users. Many genomic sequencing service providers use this model to be self-sustainable without much external funding e.g., Ramaciotti Centre for Genomics at UNSW. We believe this model can be grown but recognise that we are not a genomic service provider.
Genomic sequencing underpins many essential activities outlined here, in particular, biobanking, phage matching and therapeutic monitoring. Success in making this self-sustainable right at the beginning in stage 1 demonstrates the feasibility of this venture beyond the next 5 years.
Concluding Remarks
Fig. 6. outlines our proposed commercial development pathway, which again, highlights the importance of co-investment of public and private sectors.
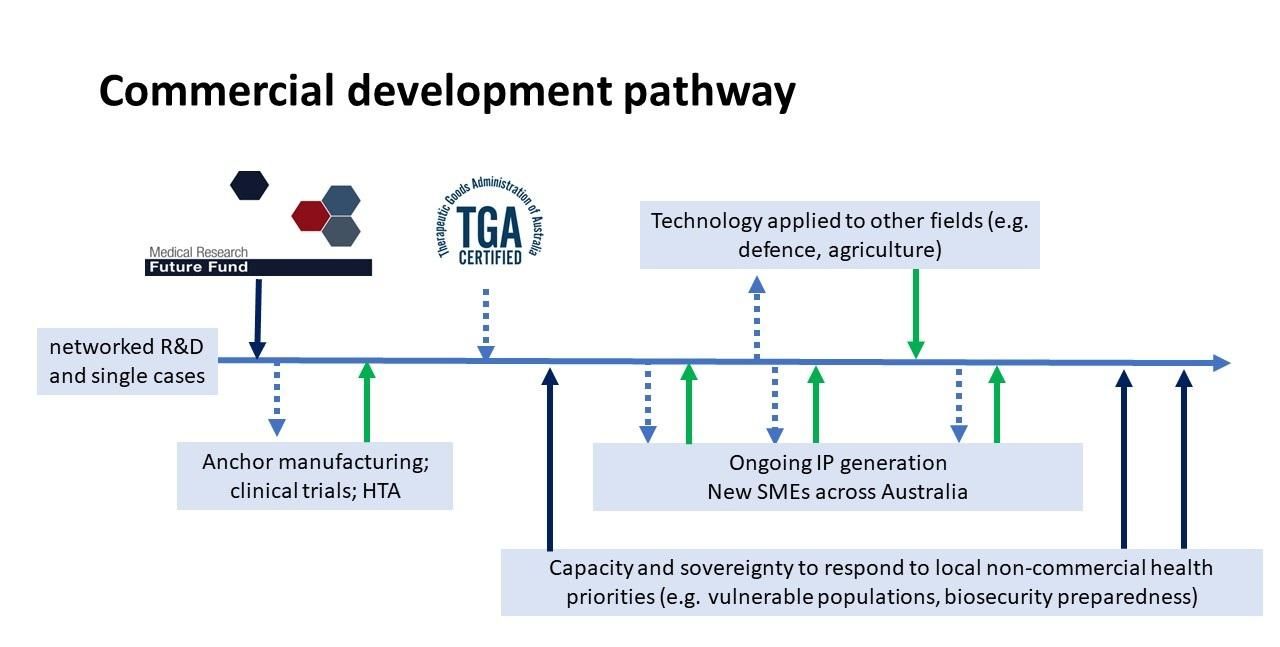
Fig. 6. Commercial development pathway of Phage Australia. X-axis denotes timeline. Dark arrows denote public good funding. Green arrows denote investment. Dotted arrows denote contribution to and from Phage Australia. HTA – health technology assessment. SME – small to medium-sized enterprise. Milestones from Stage 1 has been achieved (COVID permitting). (credit: Laura Collie)
A significant challenge facing any effort to commercialise phage therapy is that phages discovered in nature cannot be patented. It is feasible, however, to identify IP (including the know-how) along this pathway including; genetically modified phages, new cocktails containing optimal combinations of phages, new antimicrobials, chemically modified phages, AMR-cured manufacturing bacterial host strains, companion diagnostics for therapeutic screening etc (see list of income streams outlined above). From my perspective, the development timeline based on the last 12 months (Fig. 7) can be projected to increase value and de-risk the challenges I have highlighted so far.
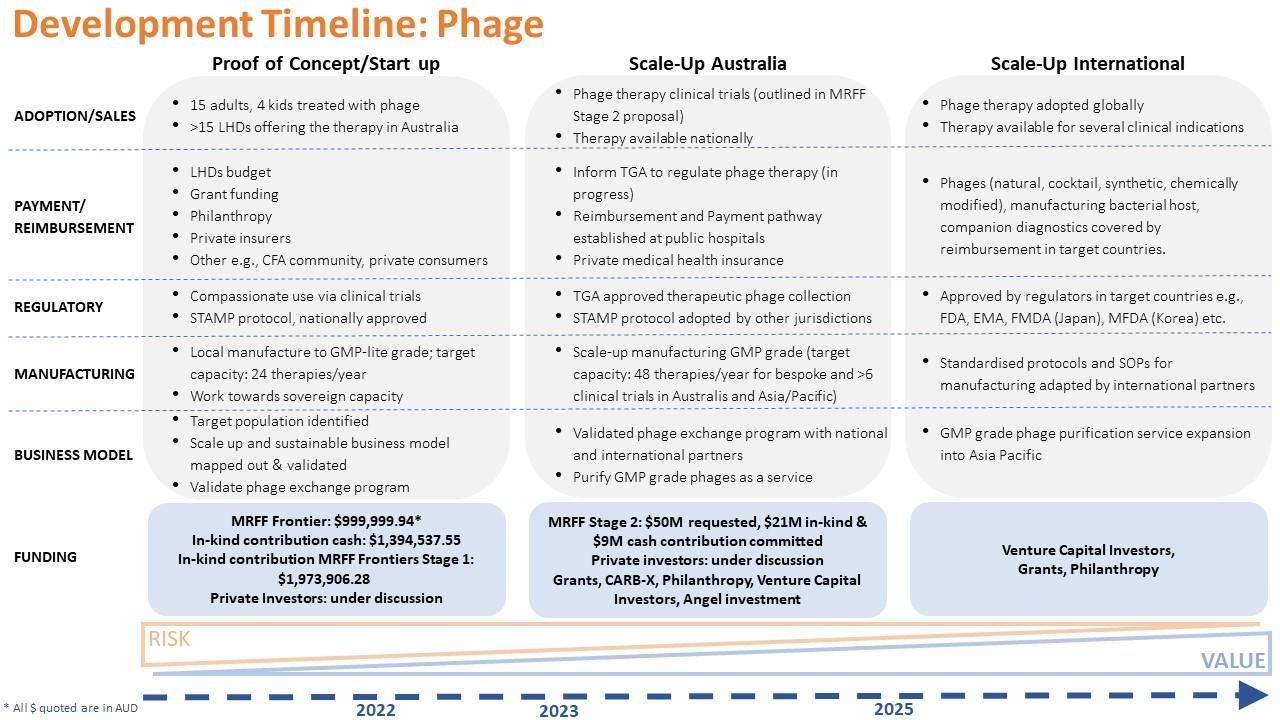
Fig. 7. Development timeline for phage therapy. The first 12 months is in real time (in line with MRFF Frontiers Stage 1 work) with risk and value projected beyond 2023. (credit: Idalia Dawidowska)
British economist Jim O’Neill was commissioned by the UK government to review AMR [10] and he identified phages as the number 1 option to antibiotics. While it is encouraging to know that there are many new and innovative preclinical pipelines of antibacterial agents [11] and antibiotics [12], given the track record of antibiotics leading to AMR, phages pose as an outstanding AMR solution, and demands immediate attention and investment from both the government and venture capital.
So, can phage make money amidst all the challenges I have outlined here?
I would like to quote Stephen Stick and leave you with this thought: “Howard Florey didn’t discover penicillin but he worked out how to use it. His first thought was not “how do we make a buck out of this”? Yet the application of penicillin created a mega industry including discovery, manufacture, formulation and delivery modes.”
References
- https://www.nfx.com/post/biotech-to-techbio
- Phage Biobank: Present Challenges and Future Perspectives. RCY Lin, JC Sacher, PJ Ceyssens, J Zheng, A Khalid, JR Iredell & The Australian Phage Network. Current Opinions in Biotechnology https://doi.org/10.1016/j.copbio.2020.12.018
- Pirnay J-P: Phage Therapy in the Year 2035. Frontiers in Microbiology 2020, 11; https://www.frontiersin.org/articles/10.3389/fmicb.2020.01171
- https://fortune.com/2022/03/31/cryptocurrency-web3-skepticism-molly-white/
- https://www.tempus.com/
- https://www.cnbc.com/2020/06/17/tempus-ai-health-firm-backed-by-groupon-co-founder-hunts-coronavirus.html
- Phage Directory: Airtable list https://airtable.com/shr8f1wbrK2hvR1an/tblCpqDQJjqyqgtj0/viw01yqq3wEoCCgie
- https://genomicmedicine.se/en/
- https://www.searchinglifescience.com/media/123/scandinavia-is-the-latest-genomics-hub
- O’Neill, J. (2016). Tackling Drug-Resistant Infections Globally: final report and recommendations, Wellcome Trust and UK Government.
- https://www.who.int/activities/coordinating-r-and-d-on-antimicrobial-resistance
- https://www.pewtrusts.org/en/research-and-analysis/data-visualizations/2014/antibiotics-currently-in-clinical-development
Downloads
PDF: Impact of Phage Therapy on AMR Sepsis, prepared by HTANALYSTS
PDF: Impact of Phage Therapy on Joint Infections, prepared by HTANALYSTS
Acknowledgement
- Discussion on commercialisation of phage and phage therapy: Jon Iredell, Laura Collie, Idalia Dawidowska, Viktor Windeyer, James Jones, Sam Walker, Jan Zheng, Jessica Sacher, Stephen Stick, Trevor Lithgow, Joey Lai, Bill Russell.
- Discussion on market sounding analysis of phage therapy, pathogen and phage biobanking, TGA working group and education package on phage therapy: NSW Health i.e., Tony Penna, Laura Collie, Julia Warning, Sian Hope, Patricia Morten, Anne O’Neill, Caitlin Pitt.
- Contribution to HTAnalyst reports: Jon Iredell, Ruby Lin, Ameneh Khatami, Indy Sandaradura, Tim Gilbey, Amit Shetty, Aldo Saavedra.
- Discussion on infrastructure, governance and operations of Phage Australia: Jon Iredell, Mark Rees, Nicholas McKay, Mark Mclean, Phage Australia executives.
- Discussion on ethics, patient screening, phage exchange, genomics workflow, biobanking and surveys: Jon Iredell, Stephanie Lynch, Joey Lai, Jessica Sacher, Brian Gloss, Jan Zheng, Lani Atwood, Anthony Kicic, Nouri Ben Zakour, Ruwani Dissanayake, Steve Petrovski, Nicki Mileham, Martin Plymoth, Ameneh Khatami, Holly Sinclair, Shinwon Lee.
- Discussion on international phage biobanking, phage exchange and AMR network: Heejoon Myung, Pieter-Jan Ceyssans, Jean-Paul Pirnay, Tobi Nagel, Ben Temperton, Graham Hatfull, Greg German, Stephanie Lesage, Richard James, Richard Alm, Ronen Hazan, Ran Nir-Paz.
- Special thanks to the research support team at the Sydney University: Ed Hendriks, Nicole Makoviney, Kailing Wang, David Boyd.
To cite the health economics report:
Phage Australia. Impact of Phage Therapy: AMR Sepsis. 2022. Available from: https://dl.phage.directory/WIMR01_AMRSepsis_WP_FINAL_26July2022.pdf.
Phage Australia. Impact of Phage Therapy: Prosthetic Joint Infections. 2022. Available from: https://dl.phage.directory/WIMR01_PJI_WP_FINAL_26July2022.pdf.
To cite this white paper:
The full white paper can be found on Phage Australia
Lin, RCY. Westmead Institute for Medical Research (2022). Phage Australia - building a sustainable ecosystem for phage therapy. Retrieved from https://phageaustralia.org/blog/sustainable-phage-therapy
NB: Please feel free to write to me with your thoughts. I extend a challenge out to entrepreneurs and intrapreneurs to help us refine business plans and business models.









