Thanks for coming back to this third post about my recent foray into phage-making! To recap, as part of my work in the Iredell Lab as part of Phage Australia, I’m working on developing a short-term ‘minimum viable process’ for producing safe phage preps for patients who need them urgently (our waiting list is now 40+ patients deep). Long term we are working on a GMP process, but for now, we are working with what we have access to: essentially standard microbiological methods.
In post #1 (How to start making phages for therapy), I shared how I went from being overwhelmed by the zillions of options to landing on a functioning first version of a ‘minimum viable process’ for phage production.
In post #2 (Therapeutic phage-making: how my first batch measured up), I shared my first steps toward evaluating that process (spoiler: somehow it worked! At least for the first phage I tried it with).
Now that we’ve got a default method of making phage and reducing endotoxin that seems to work, it’s time to decide how we’re going to really show that our phage quality is safe enough to use in a patient. But who makes the rules? How do we meet the standards?
Welcome to post #3, where I will share how I’m thinking about phage quality control these days.
Note: what follows is an informal window into my personal journey into learning about phage QC, and a bit about how I’m thinking about it as a beginner trying to make sense of a very complicated web of new concepts. It’s also only a snapshot of what will be a constantly evolving process. If you’re looking for a comprehensive guide to phage therapy QC, here’s an excellent resource: Quality and Safety Requirements for Sustainable Phage Therapy Products (Pirnay et al. in 2015)
What is safe?
At least in Australia, ‘safe’ is not yet defined for phages. Australia’s federal health regulator, the Therapeutic Goods Administration (TGA), doesn’t have an official position on them. Even if they did, it wouldn’t necessarily apply to the stage we’re currently at. We’re not trying to show a drug product is good enough to test in a clinical trial, we’re just trying to address urgent patient need right now. As with the US FDA, the rules are different when you’re at this stage.
So we can do whatever we want?
Not really.
First, the patient’s doctor needs to justify to the TGA that the patient getting phages qualifies under Australia’s version of compassionate use (it’s called Special Access Scheme Category A). (This is similar to how the FDA currently makes this call for phage preparations made by IPATH, TAILOR, Yale, etc).
Second, even though there is no official guidance on what safe means, we (the people making the phage) still need to justify to our local drug committee and ethics committee that our product is safe.
So our phages need to be ‘safe’, but no one is going to tell us what ‘safe’ means?
Essentially yes. It looks like it’s on us to:
- Decide (and justify) what to test
- Figure out how to do the tests
- Do the tests
- Show the results of the tests
- Show that the tests were done properly
Can’t we just outsource this?
Conveniently, there are companies that you can pay to test the quality of your pharmaceutical products (like Charles River or Eurofins). They presumably have all the right accreditations and know what they’re doing. And of course if we’re the phage makers, anything we do internally is going to be less credible than sending our phage to a third party to give us a clean bill of health for our phage. So outsourcing seems logical.
Over the last few months, we did reach out to a couple of companies and had some conversations. But what I learned from this experience was — surprise — WE have to be the ones to tell these companies what to test. And we have to know things like ‘what is the volume of final product you’ll have?’, ‘how many aliquots of that will you make?’, ‘is it always going to be made that way?’, and ‘what standards do you want to comply with?’. And every time you give them a new product (ie. a new phage), there is a new ‘validation’ process that needs to be done, which takes extra time and money (understandably, but still). We don’t really have these kinds of answers yet, as we’re anticipating a lot of tweaking with each new phage, having to go up in volume for phages that don’t propagate well, etc.
So it doesn’t seem like we’re ready for outsourcing.
Cool… so we’re back to square one? It does seem that way. Once we have a more defined process, we can definitely outsource, but it doesn’t make sense for us right now. For now, let’s get started tackling this on our own, and getting at least a minimal, default phage QC (quality control) pipeline set up.
What I’ll cover today
In this post, I’ll share a bit about how we made decisions surrounding the first step in my list above:
- Decide (and justify) what to test
What I won’t cover today
For now, the rest of the list is still in progress, but I hope to cover it in the future when I know more.
- Figure out how to do the tests
- Do the tests
For our first two phage preps, I’ve now done endotoxin testing and sterility testing. Will report back on these soon.
- Show the results of the tests
- Show that the tests were done properly
There are controls we’re doing in each test, of course, but beyond that, I’m learning there’s all kinds of tests that can be designed to show that a certain test is valid (’validation protocols’). This is definitely a part that the outsourcing will help us with down the road, but helpful to know about it and think about it now.
So many things we could test for, so many ways to do each: where to begin?
There’s a lot of things you could do to demonstrate safety of a phage prep. And lots of ways to measure each one. For instance, we might sequence the phage prep at the end to make sure there’s only that one phage in there, and no trace of the host bacteria or any other contaminating bugs that may have gotten in. We might also culture a sample of the phage prep (a sterility test) so we can be more confident that it’s sterile. And we might measure the pH to make sure it’s close enough to what’s physiological, so it doesn’t shock anyone’s system. But what about toxins or components that don’t come from the phage, but rather from the host?
This sounds like another problem like the one I described in my first post of this series… a dizzying array of things to do, and a multitude of ways to do each. Where to start? I decided to apply the same decision-making framework I used in post #1, where I start by reminding myself what I’m optimizing for, then I break the problem down into components, look at my options for each, filter for materials/expertise I have access to, make a choice of one thing to try first to address each step/component, and document my reasoning. Then I come out with a non-overwhelming starting set of steps I’m happy with that address everything I need to address.
What does success look like? (What are we optimizing for?)
We need phage that is safe enough to satisfy our local drug and ethics committees, and the health regulator (TGA), but for now we don’t exactly know what they would want. But we do know regulators tend to look to other regulators (what are other governments happy with?). So it seems like a reasonable strategy to start with looking at what others do to show their phages are safe, copying them as much as possible, and focusing on documenting it well so decision-makers can make their decisions with confidence.
Importantly, we are optimizing for getting our phage product approved for compassionate use, and NOT optimizing for a perfectly safe phage prep (since we are trying to address immediate patient need). We don’t have a lot of time or specialized equipment, and we have a lot of unknowns. And we know the decision-makers are going to factor in the fact that doing nothing, or taking too long to define a perfectly, guaranteed-to-be-safe phage product, will be worse for the patient. So we need to show a good level of safety without taking too long to get there.
Making a list of dangerous things we could test for, and how we might test for them
Our phage prep is going to contain things that are potentially risky or dangerous. Let’s consider that ‘safe’ means lacking dangerous features. What could be dangerous? (To compile this list I looked at what others seem to be testing for, eg. Sciensano, Belgium’s health authority, which has a phage QC department, and TAILOR labs, who provide phages for US compassionate use cases. I also talked to Jon, my advisor and Phage Australia’s leader, about what might scare him as a clinician.)
We realized there are three main ‘sources’ of dangerous components: the host, the phage, and the final product (the batch). For each, I’ve listed one or more possible solutions we’re considering (and/or some notes on how we’re thinking about it).
Risks from host
Problem: Genes from the host bacteria (like prophage genes, AMR genes, virulence genes)
Solution — Sequence the host: we have access to sequencing and a bioinformatician to analyze the results and come up with a list of ‘red flag’ genes
Risks from phage
Problem: Genes from the phage (like lysogeny genes, AMR genes, transduction genes)
Solution — Sequence the phage
Risks from final product (the batch)
Problem: Bacterial contaminants
- Solution — Test for sterility: We are next door to an accredited hospital diagnostics lab, which routinely cultures microbes from patient samples in blood culture bottles; if we inoculate them with our phage preps, they can culture them and let us know if anything grows
- Solution — Sequence the batch
Problem: Components from the bacterial lysate during production (like endotoxin, exotoxins)
- Solution — Quantify endotoxin: we can quantify this using the Charles River LAL (limulus amoebocyte lysate) system (we have one at our institute)
- Solution — Detect known toxin genes by sequencing: Sequencing the host should tell us (at least about known toxins). Also, we’re using fairly benign strains, so this helps reduce this risk too.
- Solution — Detect unknown harmful factors by cell viability assay: (as done by Luong et al.) Right now we aren’t set up for this but would be good to look into for the future
Problem: Overly acidic or overly basic pH of the product
Solution — Measure pH: we will check and aim for physiological pH (our phage sample will be diluted 10x-100x or more in standard saline, so presumably this won’t be too big an issue)
Problem: Octanol (which we introduced during the production process)
Solution: We don’t currently have a method to test this, but others (Barr & Rohwer labs, Patterson case) have safely used octanol-purified preps in patients (we will likely submit something similar to the risk assessment they did; many thanks to Jeremy Barr for advising us on this and sharing tips and info!).
Note: We do know that octanol inhibits the endotoxin test, but we’ve seen that our endotoxin test’s standards work in presence of our phage prep, suggesting octanol levels are low — so this at least suggests that our dialysis step removed most of the octanol, as it should have (see Phage on Tap protocol and blog post #1 and #2 in this series)
Problem: Other phages (prophages or contaminating phages)
Solution — Sequence the batch
Narrowing the pool and making choices
Next, in line with my framework for preventing overwhelm and optimization creep, and to identify gaps in my thinking, I’ve started filling out the table below to document my options, choices, and reasoning:
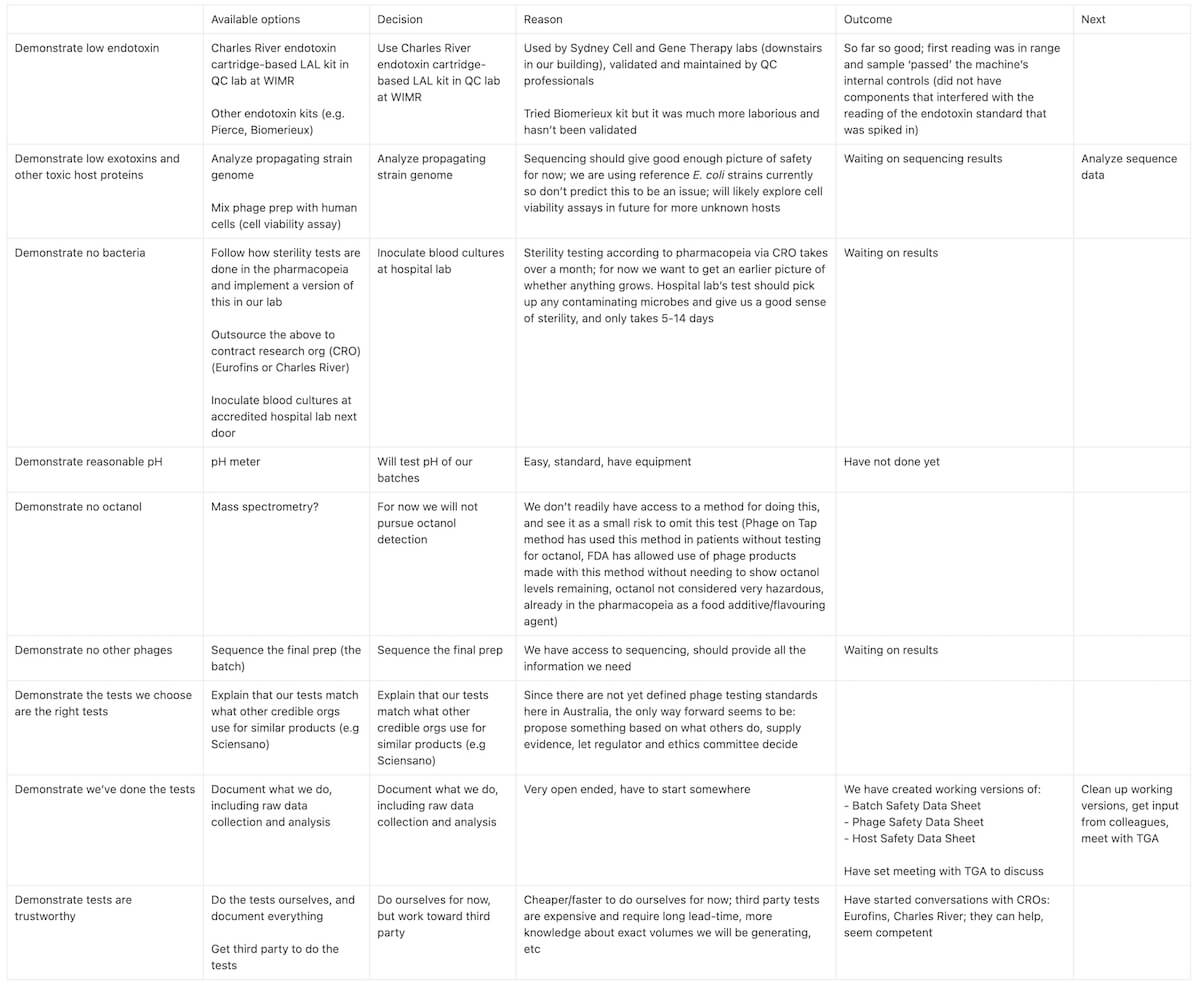
Table 1. For each ‘dangerous’ potential component we’ve identified could be in a phage batch, I’ve filled out some options for testing it, decisions we’ve made, and our reasoning as our team works toward setting up a basic phage quality control pipeline in the lab.
Decisions made: Next, carry them out
For most dangerous components we’ve identified, we’ve decided on a test, and we’ve gotten clear on our reasoning. For now, our ‘minimum viable phage QC pipeline’ looks like:
- Endotoxin test using LAL assay (Charles River cartridge+machine)
- Sterility test using blood culture bottles (hospital lab)
- pH test using standard pH meter (our lab)
- Sequencing the phage, the host and the final batch (sequencing center in our institute)
- Risks we’re not testing for right now: octanol, unknown toxic components (because we believe the risk of the patient having to wait until we can do these tests, which we are not set up for, to be higher than the risk of letting the patient have the prep without these components being tested for, given we believe they are quite low risk)
- Document everything and submit to local drug committee, ethics committee, TGA
I’ve started doing these tests on two phage products I’ve now made (both E. coli phages), which we’re preparing for a patient at the hospital next door. Once I finish the set of tests we’ve decided on, I’ll be compiling the data and methods, and submitting them to our drug committee and ethics committee (with lots of help from others here who’ve done it before).
In a future post I hope to update you on how these assays went. I will also eventually talk more about the way we’ll be documenting everything, and of course, whether our first preps end up making it through… I am assuming we’ll get feedback and need to make some tweaks, and I’m excited to see what that looks like.
Thanks for reading! As always, I love your comments and emails, please keep sending your thoughts if you feel like it. Especially if there are things I’m missing, or my logic is flawed, or you think there’s an easier way for me to address anything I’m trying to do. Thanks in advance!
A few meta-reflections as I’ve delved into this new area
Unexpected source of help: People at my institute that DON’T work on phages
In my quest for setting up this minimum viable phage QC pipeline, one of the best things I’ve done is having lots of conversations with others in our institute (Westmead Institute for Medical Research) who are working on everything but phage.
For example, there are people upstairs who make CAR-T cells for cancer therapy, and who do things like islet cell transplants for diabetes therapy. There are also people in the neighbouring building who are making viral vectors for gene therapy. All of these end up needing to develop a quality control pipeline that from what I gather, looks a lot like what we need — and they all seem to have started in academic labs, which is encouraging!
So I’m very excited to learn as much as I can from them, and so far it’s been extremely helpful. (e.g. I ask around and set up coffee meetings with T-cell makers and bone marrow researchers and QC managers in my building, etc, and people have been more than happy to help me learn about GMP, QC, and tell me about their process — shoutout to Natasha Barry, Flora Kan and Leighton Clancy especially!)
Quality control and manufacturing might actually not be boring
As someone who used to think words like ‘GMP’ and ‘manufacturing’ and ‘QC’ were the most boring words possible, I am actually feeling genuinely excited about them these days… I think because I’m starting to see how important it is to get these right, and also how much creativity it actually takes to set these systems up (as opposed to being something that was already solved, or requiring long manuals to read and paperwork to fill out). I’m sure the latter are true, but it’s actually seeming more like a craft than an assembly line, the more I learn about it. And it feels very important to get right. (Now that it feels like if we mess up, a patient could have a bad outcome, I’m hyper focused on documenting things, giving my buffers batch numbers, etc, in a way I never was before).
Getting good at this stuff is probably a transferable skill!
I’m getting the sense that new biological entities are getting turned into therapeutics more and more, and there seems to be a lot of focus on getting GMP production happening in a pseudo-academic setting, rather than waiting until pharma is ready to take the reins… very interesting! Maybe a new in-demand skillset for our future careers?
Resources that have been helpful
Sciensano, Belgium’s federal health regulator, has put some rules around what constitutes ‘safe’ for phages, and even has a phage QC lab; we are following their lead on what makes sense to test
- Magistral phage paper (2018), their reference document on how a safe phage should be prepared
- Sciensano website
- Many thanks to Pieter-Jan Ceyssens and Mathieu de Jode at Sciensano for helpful conversations and for sharing your methods!
TAILOR makes phages to US FDA standards for compassionate use: they create a ‘physician desk reference’ for their phages and describe how they make/test them:
- Paper by Austen L. Terwilliger et al. 2020
Methods papers we’re going back to again and again these days:
- Phage on Tap (paper by Bonilla et al. 2016): where we got the octanol method and so much more!
- Luong et al. 2020 paper (we use lots of their methods for phage production, QC, etc)
A comprehensive look at phage therapy QC and all the factors that should be considered, published by Pirnay et al. in 2015









