We were recently invited by CARB-X to re-publish a Spotlight article written by Robin Berghaus on SNIPR Biome, a phage therapy company funded by CARB-X. Read the original post here.
This article was originally published on CARB-X on May 16, 2023.
As SNIPR Biome completes its Phase 1 clinical trial, the company recently published new data in Nature Biotechnology about how it developed SNIPR001, its first clinical drug candidate designed to prevent bloodstream infections in cancer patients.
Infection is the second leading cause of death for cancer patients. Powerful treatments like chemotherapy can weaken immune systems and damage intestinal linings, enabling bacteria to enter the bloodstream. Among these include E. coli, a top global health threat. While E. coli normally exists peacefully in the gut, it can become pathogenic in the bloodstream. Without robust immune systems, cancer patients face a greater risk of dying from these infections.
Copenhagen-based SNIPR Biome has engineered SNIPR001, a cocktail of viruses called bacteriophages (or simply “phages”) to address this unmet medical need. SNIPR001 phages act like sharpshooters that precisely target E. coli in the gut before these bacteria can jump into the bloodstream. Precision killing can help spare a patient’s microbiome. This contrasts with traditional antibiotics–which kill a range of bacteria, good and bad.
Antibiotics are the go-to drugs that doctors have prescribed for decades, because they can treat a variety of bacteria and clear infections quickly. But indiscriminate killing may also have adverse effects, which is why product developers, like SNIPR Biome, are considering more focused approaches. Beneficial bacteria provide many services–from aiding digestion to synthesizing vitamins. And while antibiotics act broadly, they won’t clear them all. Bacteria quickly evolve to resist the drugs meant to kill them.

SNIPR Biome’s co-founder and CEO, Christian Grøndahl, Dr. Med., and Camilla Schoopp Kristensen, PhD candidate, view E. coli colonies on an agar plate. Photo by Jakob Helbig
Engineering CRISPR-armed phages
As standard of care to prevent and treat infections in cancer patients, doctors often prescribe fluoroquinolones, an antibiotic class that is becoming less effective as resistant rates rise. To help eliminate fluoroquinolone-resistant E. coli strains, SNIPR Biome developed its precision SNIPR001 platform using CRISPR, a breakthrough gene editing tool.
While first discovered in 1987, CRISPR was identified years later as a natural immune system for bacteria to keep invading viruses at bay. But CRISPR’s many applications–from curing genetic diseases to creating drought-resistant crops–weren’t imagined until 2012 when a seminal paper was published in Science. CRISPR’s ability to edit genes faster, easier and cheaper than any other existing tool was so transformative, two of the scientists who discovered its applications received the Nobel Prize.
CRISPR functions like a word processing program, but it cuts, copies and pastes genes instead of words. It works by using two components. The first are CRISPRs, short repetitive DNA sequences called “Clustered Regularly Interspaced Short Palindromic Repeats.” The second are Cas (“CRISPR-associated”) proteins, which cut DNA like scissors.
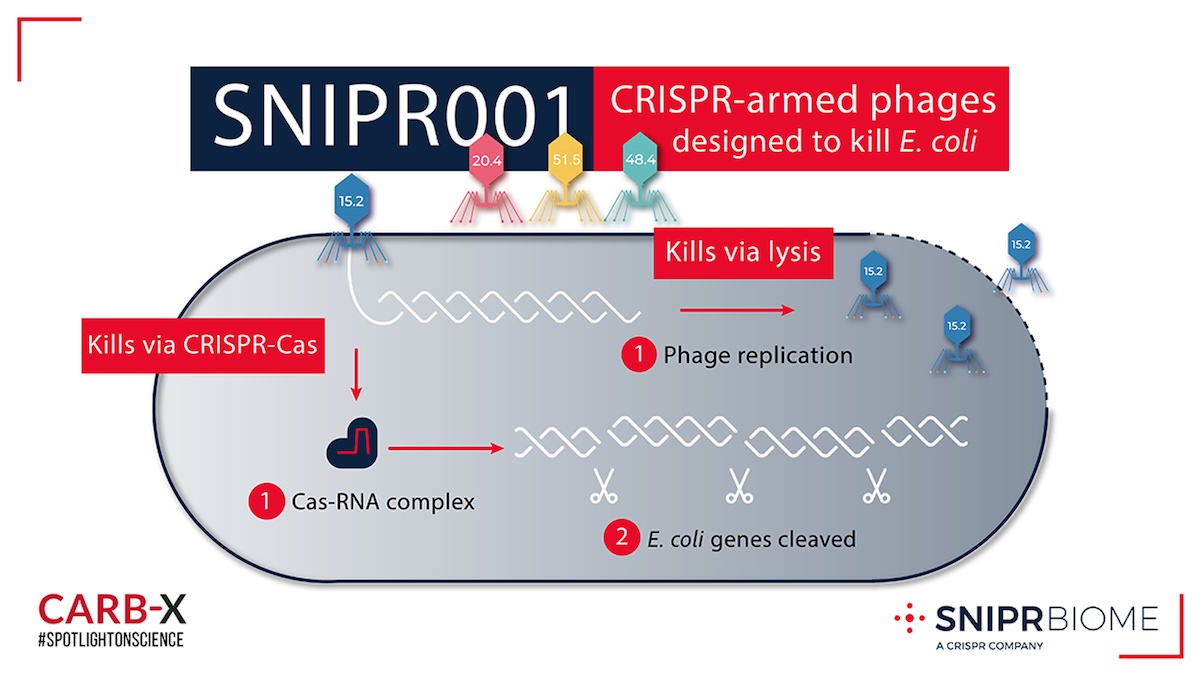
SNIPR Biome engineered CRISPR-Cas systems into phages that can explode bacterial cells. Phages do this by injecting their DNA into bacteria. Once inside, the phage DNA is replicated into armies of phages that burst the bacteria. Because bacteria often develop resistance mechanisms to prevent this, SNIPR001 provides another tool to disarm E. coli. Its CRISPR-Cas system finds and chops specific DNA sequences within E. coli to prevent them from multiplying.
Because bacteria naturally evolve to evade the drugs meant to kill them, SNIPR Biome engineered a cocktail of four phages to extend the lifespan of its drug.
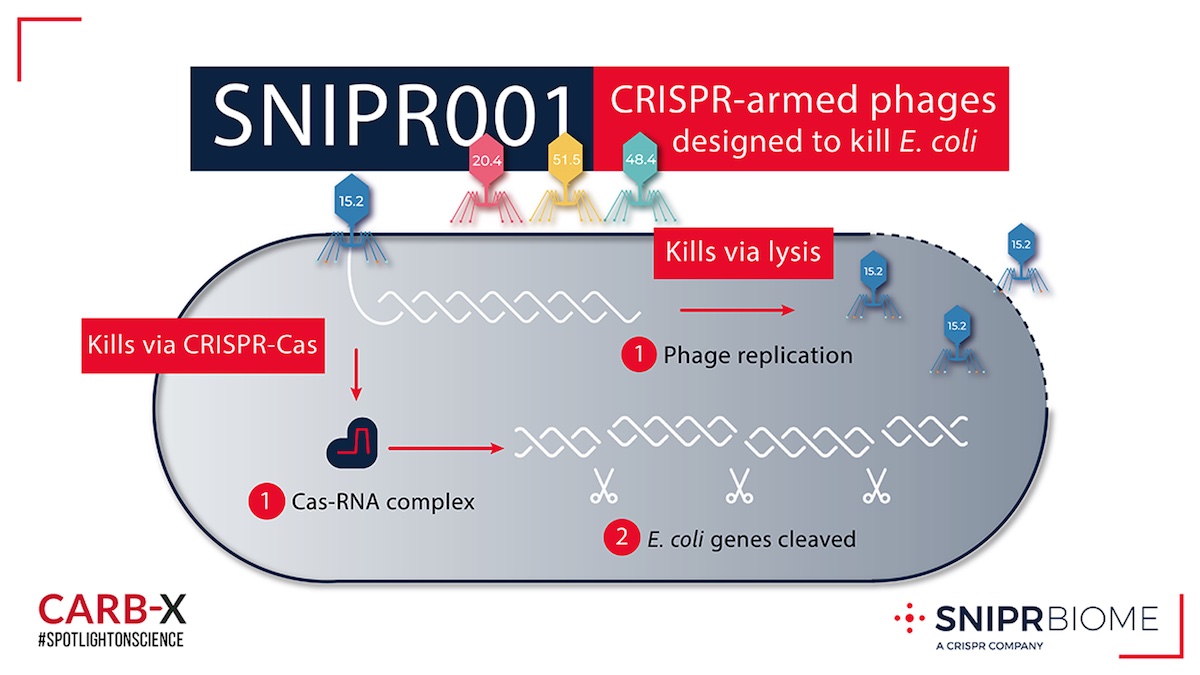
SNIPR001 is a cocktail of CRISPR-armed phages (CAPs) designed to kill E. coli. The CAPs encode a CRISPR-Cas system that specifically targets genes unique to the E. coli genome thereby killing the bacteria.
“Our initial thought was that we need to be specific and only take out the problematic E. coli strains,” said Eric van der Helm, PhD, Vice President of Scientific Affairs, SNIPR Biome. “But actually, talking to the physicians, we learned that any E. coli is basically detrimental for this patient population.”
SNIPR Biome tested a panel of 160 wild-type phages against 300 E. coli strains isolated from cancer patients. The team observed that some phages were highly specific while others acted more broadly to target E. coli. They customized phage tail fibers, elongated proteins that recognize a bacterium’s cell surface. By swapping tail fibers from one phage to another, they created phages with broader killing capabilities.
Some of these phage tail fibers recognize E. coli cell surface proteins that are highly conserved and perform critical functions for the bacteria, such as taking up small molecules needed for growth. Because they are essential, SNIPR Biome believes they may not evolve as quickly and could be reliable targets. SNIPR001 phage tail fibers also recognize lipopolysaccharides (LPS), sugars that protect bacteria from the environment. Van der Helm equates LPS sugars to “fashionable coats that can change with the seasons,” because they are prone to mutations.
By engineering the tail fibers to recognize both highly-conserved receptors and LPS targets, SNIPR001 results have been promising. Among the four resulting CRISPR-armed phages, they found most E.coli strains were hit by two or three, while half of E. coli were hit by all four.
“Our data show that you can combine these functionalities, which is really cool,” says Van der Helm. “Because it means that you can rationally design these phages as a drug, and you are not completely relying on an opportunistic screening of a large library. That makes this process scalable.”
In April 2022, SNIPR001 entered first-in-human clinical trials. During the Phase 1 trial, 36 subjects were dosed with the SNIPR001 phage cocktail and an antacid that increased the gut pH, making the harsh environment more hospitable to phages. They enrolled subjects with healthy guts and a significant E. coli population that would potentially enable them to calculate whether SNIPR001 had a measurable effect. Data from the trial are currently being analyzed, and are anticipated to be published later this year.

Photo of Eric van der Helm by Martin Søby
A novel product provides hope and challenges
SNIPR Biome entered uncharted territory as it approached clinical trials. “For a CRISPR-armed phage, you cannot go to FDA.gov and download a 40-page guideline, like you can for a small molecule to treat a UTI,” says Van der Helm.
While phages have been used in Eastern Europe for more than 100 years to treat bacterial infections, no phage therapies have been approved for use by US nor EU regulatory authorities. Unlike traditional antibiotics, phage therapies have not yet undergone the same type of rigorous randomized controlled clinical trials. If you were to search “phage” in ClinicalTrials.gov, you would find approximately 80 trials that are in various stages. In contrast, a search for “antibiotic” would return more than 18,000 studies.
Without a clear road map, the approach to clinical trial design is where Van der Helm believes CARB-X support was most transformative. Throughout product development, SNIPR Biome met monthly with CARB-X’s Advisory Board and in-house R&D team. During the Phase 1 clinical trial, they met biweekly. “There’s a lot of value coming out of these meetings,” says Van der Helm. “These are experts who could help us avoid pitfalls by advising our clinical trial protocol, including which measurements and sequencing technologies to use.”
Because phage drugs could open new ways of preventing and treating resistant bacterial infections, CARB-X sees a major value in supporting SNIPR001, and it has awarded SNIPR Biome approximately $8 million toward the development of its technology.
“SNIPR Biome’s approach to preventing breakthrough infections of harmful bacteria in compromised patients is new. Nobody knows the clinical benefits and the right endpoints,” says Erin Duffy, PhD, CARB-X Chief of Research and Development.
“SNIPR001 could be a true pathfinder, enabling us to explore key questions that could unblock the path, not only for SNIPR Biome’s product approval, but that could open the field for others. And the more unique the approaches to antimicrobial resistance, the more likely we are to deliver long-lasting treatments for drug-resistant bacteria,” says Duffy.
A CRISPR platform expands therapeutic possibilities
SNIPR Biome was founded in 2015, three years after the scientific world discovered CRISPR’s potential. While other scientists began using CRISPR-Cas to alter the human genome, SNIPR Biome sought it to control bacteria–turning their native immune systems against them. Co-founders Christian Grøndahl, PhD and Jasper Clube tapped Van der Helm who was using CRISPR in his research as a postdoctoral fellow at Technical University of Denmark (DTU). With his advisor Professor Morten Sommer, PhD and others, Van der Helm designed the early CRISPR-Cas studies which eventually led to the company’s first patent.
DTU incubated SNIPR Biome’s research for one year. The company then transitioned into its own laboratory and current headquarters in Copenhagen. Since then, SNIPR Biome has grown from 4 to 60 employees, including 40 scientists. Van der Helm believes their international footprint, which represents 20 nations, has contributed to the team’s creativity and ideation.
Team lead and lab manager, Ana de Santiago Torío, examines bacterial colonies that have been exposed to SNIPR001. Photo by Jakob Helbig
Beyond SNIPR001, the company envisions its CRISPR-Cas platform will have a range of applications, and they are engineering CRISPR-Cas systems into phages and bacteria.
“Bacteria are factories in themselves. They can replicate and make their own energy, and they have large genomes, typically 50 times larger than phages,” says Van der Helm.
These qualities enable bacteria to deliver an array of compounds–from antibodies to hormones. So Van der Helm believes bacteria could be ideal vectors to treat chronic conditions, such as diabetes and inflammatory bowel disease, where a sustained release is desired. For acute diseases, Van der Helm prefers phages, because they need a host to divide and phages clear patients’ systems quickly.
Within its pipeline, SNIPR Biome is developing a therapeutic to directly eliminate bloodstream infections, and a product that targets bacteria known to be correlated with colorectal cancer. The company also hopes that by engineering live bacteria and phages to produce therapeutic compounds, they could eliminate time-consuming manufacturing and help drive down costs for patients.
Eric van der Helm — Vice President of Scientific Affairs

Photo of Eric van der Helm by Martin Søby
Eric van der Helm is a biotechnology R&D leader with experience in synthetic biology, drug discovery, microbiome research, and bioinformatics. After completing his undergraduate studies in the Netherlands, he joined the research group of David Baker at the University of Washington (UW), where he focused on the computational design of protein-protein interactions involved in cancer immunotherapy.
Van der Helm earned his PhD in the Sommer lab at the Technical University of Denmark (DTU), where he developed a novel platform for drug discovery using genetically engineered bacteria.
For the past six years, he has played a pivotal role at SNIPR BIOME as VP of Scientific Affairs, Automation & Bioinformatics. In this capacity, Van der Helm has established multiple departments and worked on the development of the company’s microbiome modulation platform using CRISPR-Cas. He has also led the CARB-X funding application for SNIPR001 and spearheaded the company’s first publication on its technology in Nature Biotechnology.
For more information, sniprbiome.com
Published on May 16, 2023