Every development process in an innovative biotechnology or biopharmaceutical plant has an identical goal: to bring a new bio-based product to market while taking into account acceptable costs and maximizing profits. One of the biggest sources of the cost of goods is the production process itself, and therefore also the type of technology used in this process.
Traditional bioproduction plants rely on stainless steel (SS) fermenters with large capacities (20,000 L or more) and related downstream processing equipment. In the mid-2000s, the dominant status of the reusable bioprocess technologies encountered competition from the first single use (SU) fermenters.
At this point, a dilemma emerged as to which type of technology to choose, and which one would be the most efficient and suitable for a given bioprocess. Although disposable fermentation systems are still very limited in scale compared to stainless steel systems (the largest disposable systems currently reach around 5,000 L), they are more often used as equipment in innovative biotechnology labs than difficult to use and expensive to upkeep SS systems.
As a representative of a manufacturer of SU systems, the first question I encounter most often is how to compare the costs of SU systems to more traditional reusable solutions. Undoubtedly, anyone considering switching to a SU system immediately notices the cost of consumables. Mostly, they indicate that these costs are very high, and fermentation in a steel bioreactor requires virtually no operating costs. They also say that the disposable materials generate huge amounts of waste. This reaction is perfectly logical and understandable; however, the reality is surprisingly much more complex.
Compared to SS solutions, the main advantage of the SU technology is the absence of cleaning and sterilization in place (CIP/SIP) normally required between processes in each of the SS devices. This routine activity takes a long time and requires the instruments to be turned off, frequently for several days at a time and requires the system to be certified for sterility prior to being used again. Switching to a SU system, CIP is minimal, SIP is entirely removed, and the duty of sterility validation is transferred from the operator to the equipment manufacturer.
In addition, SU technologies allow for a much greater scope for adapting the laboratory to conduct various bioprocesses, excluding the danger of cross-contamination. For example, a bioprocessing laboratory outfitted with reusable equipment is usually dedicated to only one type of bioproduct, therefore, the production of various preparations requires construction of multiple production lines.
On the other hand, using SU technologies we can completely replace all components of the production line, which come in contact with the process, with new ones, and thus completely separate the processes despite using the same equipment. By avoiding the cleaning of equipment between batches, we also save on staff work time, who can focus on the production instead of equipment maintenance.
The costs and profits analysis of SS and SU technologies is also very interesting. The highest costs of SS technology are the cost of purchase and installation, followed by the cost of CIP/SIP [1], including labour time and costs. SU solutions have significantly lower capital costs, up to 40% [2, 3], and a much faster lead time for delivery of capital equipment, which allows for more time to make the final decision on which particular SU solution will be used in the newly created laboratory or processing plant. In addition, SU systems reduce operating costs by up to 20% and employee costs by a further 10% [4]. Of course, these costs are partly offset by high consumables prices, however, on balance the costs of the energy required to maintain a SS system outstrips the consumable costs of SU.
The potential profits should also be considered in the above calculations, as well as costs of goods. For example, according to a recent study, the profits from the production of 2000 L of monoclonal antibody suspension in a SS system in comparison to a SU system, in which not only the bioreactor system was replaced with a SU solution but also the centrifuges were replaced by filtration systems, the SU system generated 91kg of bioproduct at a total cost of 70 Euro/g. The SS system, on the other hand, yielded only 87kg of product with a cost of 102 Euro/g [2]. According to the authors of this study, the main sources of difference in the cost of goods are the product losses during downstream processing and the maintenance costs of the reusable equipment, including chemicals and deionized water used for CIP/SIP.
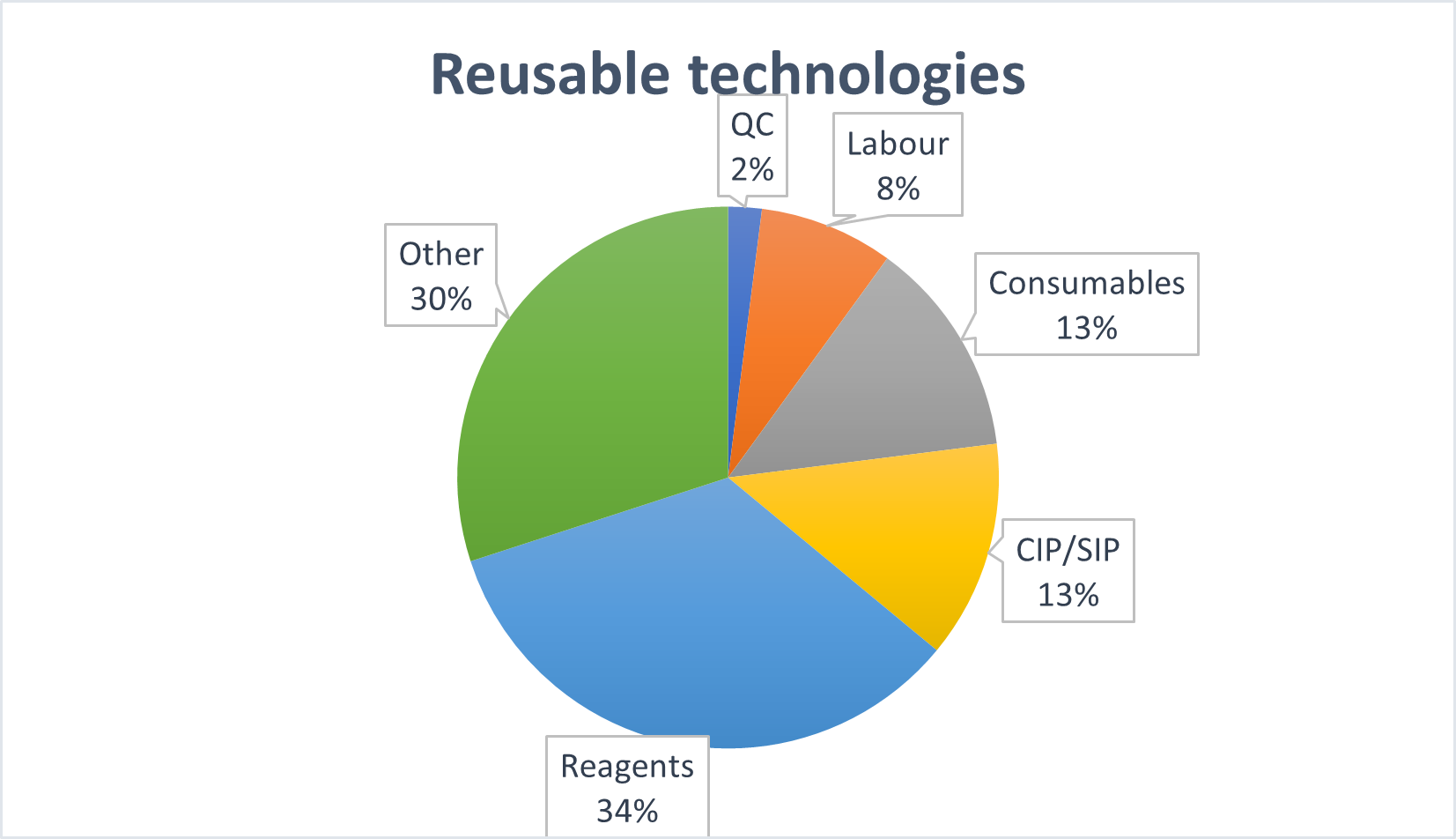
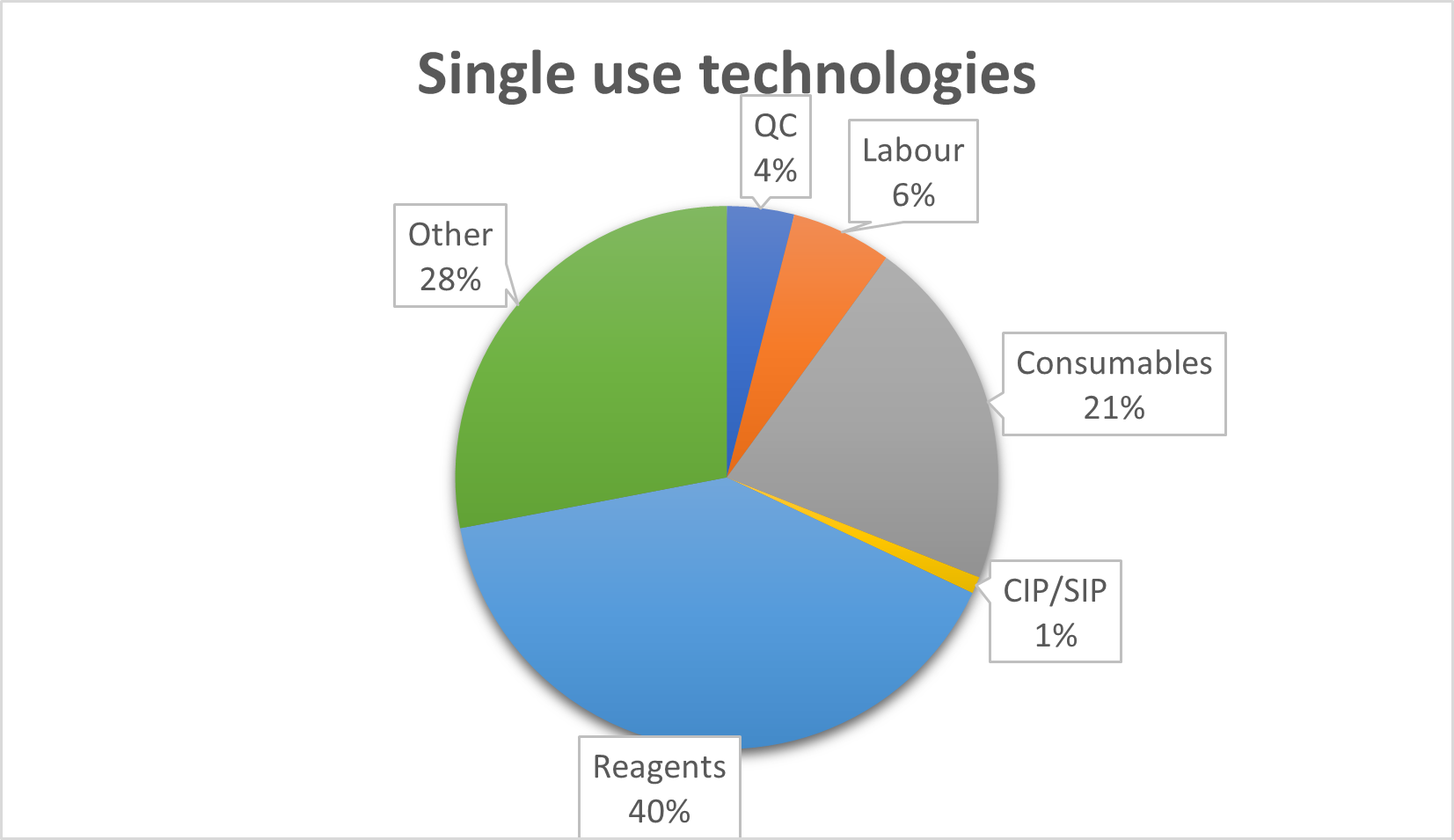
Figure 1. Proportional comparison of costs of product using single use or reusable technologies at production scale of 3000 kg of bioproduct per year. Based on Mahal et al. (2021).
In addition to financial costs, environmental costs are also inevitable in any bioprocess. SU technologies generate large amounts of plastic waste. Their presence is very visible, and the sizes can be overwhelming. However, a well-managed bioprocess plant can use the services of incineration plants, where some of the energy used for the production of consumables can be recovered as heat from incineration of the waste material [5]. In addition, the cost of energy, highly toxic chemicals necessary for CIP/SIP processes, their disposal and production of deionized water necessary for cleaning of the machinery, is often hidden in indirect costs. These can amount to even 13% of the total production costs on a scale of 3000 kg of the product per year or even more at smaller production scales. In comparison, the cost of combustion of the SU consumables can be significantly lower than the CIP/SIP costs [1,3,5].
In summary, the answer to the question of which technology to choose, reusable or disposable, is not a straightforward one. Such a decision should be based on the type of bioprocess being performed, the certification requirements and the needs of the bioprocessing plant. When choosing an SU technology provider, we choose not only a contractor, but also a partner for a long period. The SS solution provider, similarly, should be able to guarantee long-term service of the equipment. Undoubtedly, however, SU technologies are more flexible and adaptable, and particularly useful where the ability to quickly switch to new requirements is crucial, and your equipment is used for a wide range of applications both upstream and downstream.

The Cellexus CellMaker.

Proteon Pharma’s lab, which features Cellexus CellMakers.
References
-
Mahal, H., Branton, H. & Farid, S. S. End-to-end continuous bioprocessing: Impact on facility design, cost of goods, and cost of development for monoclonal antibodies. Biotechnol. Bioeng. bit.27774 (2021) doi:10.1002/bit.27774.
-
Gupta, P., Monge, M., Boulais, A., Chopra, N. & Hutchinson, N. Single‐Use Process Platforms for Responsive and Cost‐Effective Manufacturing. in Single‐Use Technology in Biopharmaceutical Manufacture 201-210 (Wiley, 2019). doi:10.1002/9781119477891.ch16.
-
Guldager, N. Cost advantages of single use technologies. Pharm. Technol. 34, 26-31 (2010).
-
Lütke-Eversloh, T. & Rogge, P. Biopharmaceutical manufacturing in single-use bioreactors current status and challenges from a CDMO perspective . Pharm. Ind. 80, 281-284 (2018).
-
Flanagan, W. et al. An Environmental Lifecycle Assessment of Single-Use and Conventional Process Technology: Comprehensive Environmental Impacts. BioPharm Int. 27, 40-46 (2014).
Many thanks to Stephanie Lynch, Atif Khan and Tolulope Oduselu for finding and summarizing this week’s phage news, jobs and community posts!










