A Global Vision of an Infection-free Humanity
The threat of antimicrobial resistance (AMR) looms large over our global healthcare system, posing a significant challenge of what once was a simple treatment of wound or illness to battling life-threatening infections. A paper recently published by The Lancet estimates 5 million deaths each year are associated with drug-resistant bacteria, which is projected to only get worse [1]. The economic impact of this crisis is staggering; by 2050, AMR could cost the world $100 trillion in lost economic output [2]. Far more frightening are the nearly 10 million deaths annually which are expected to be a direct consequence of AMR bacterial infections by 2050, a number that is estimated to exceed deaths caused by cancer. Recent epidemiologic evidence suggests these numbers may be a reality sooner than expected, with over 2.8 million AMR infections occurring in the US alone in 2022 [3]. With antibiotic discovery and development disincentivized by antibiotic resistance and the continual occurrence of “super bugs”, the medical community faces the ongoing global health crisis dubbed the “silent pandemic”.
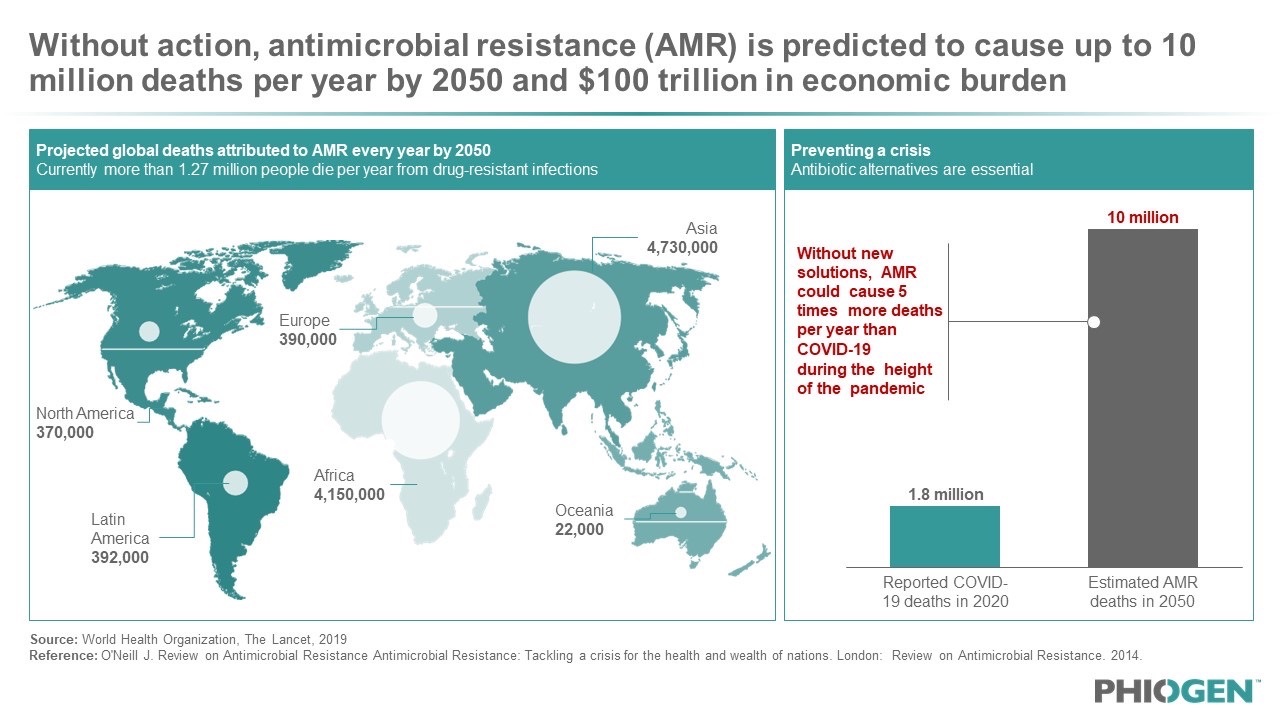
Figure 1: The WHO has deemed the AMR crisis the “silent pandemic” that is projected to cause more than 10 million deaths by 2050, five times as many losses as what was seen during the height of the COVID-19 pandemic.
At the core of this catastrophe is bacterial evolution and the inability of antibiotics to adapt. Because they are static in structure, antibiotics are limited in chemical space. Once resistance occurs, a new drug must be developed, costing on average $1 billion and 20 years to get to market approval, only to see bacterial resistance emerge and the new compound succumb to the same failures as its predecessors. Thus, there is an urgent need to find next-generation antibacterial solutions that can be adapted in real time and overcome the colossal hurdle of AMR.
Biotech startup PHIOGEN, launched in June 2023, has developed a business model that is economically scalable, investable and bridges regulatory pathways; most importantly it has established a technology platform that produces therapeutics which address the scientific and biologic challenges bacterial pathogens use to undermine traditional medicines. The company is a spin-off from Baylor College of Medicine, born out of innovation from the globally renowned research team at TAILOR Labs, the United States’ only academic phage therapy center that is actively screening and producing phages for patient use. At least 24 patients with serious infections have been treated with phages from the center under the FDA’s emergency use authorization. PHIOGEN’s headquarters is located at the world’s largest medical complex inside the prestigious Texas Medical Center’s Innovation Hub in Houston, Texas.
Premium Phages from Discovery to Delivery
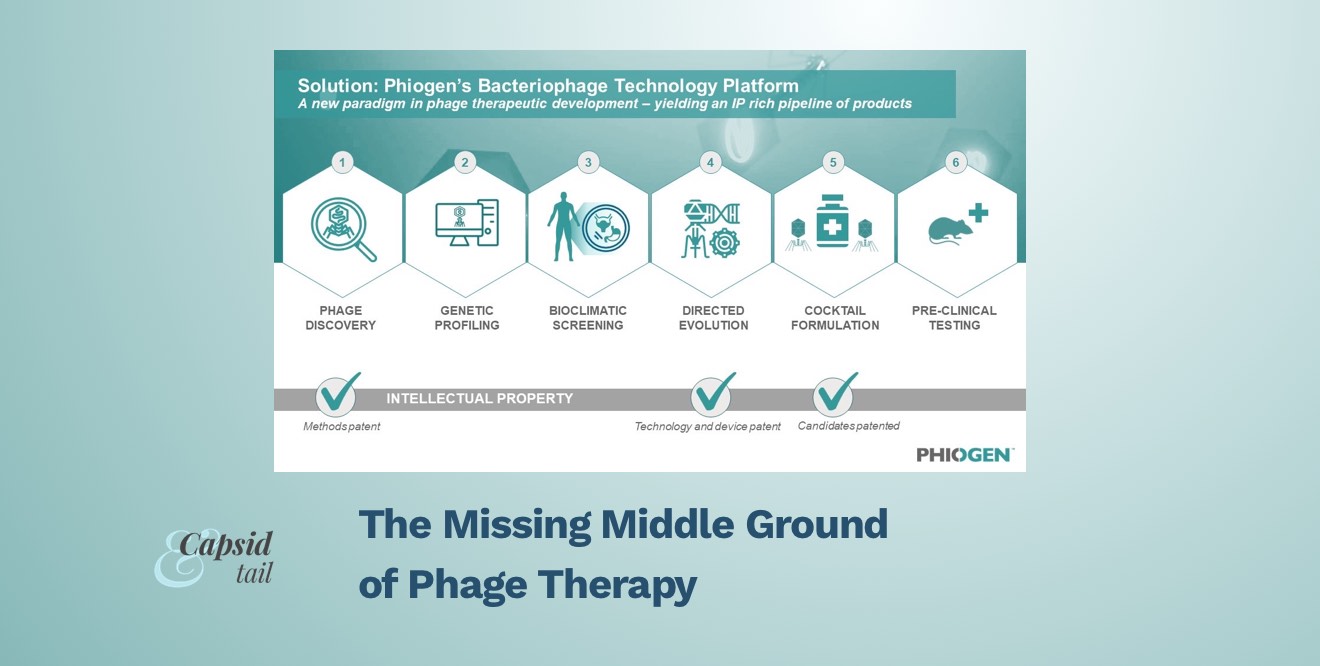
Leveraging the learnings of the Emergency Investigational New Drug (EIND) cases treated by TAILOR Labs, PHIOGEN has built a world-first technology platform that mobilizes the natural power of bacteriophages to tackle the myriad of problems bacteria use to undermine medicine, including resistance. The segmented technology platform consists of six distinct modules which filter and validate phages during each step of the process, from discovery to delivery. This approach prioritizes identifying only the most elite bacterial killers among pools of millions of phages, de-risking these phages through bioclimatic screening, evolving anti-resistor phages and developing “smart cocktails” which consist of formulations that work synergistically together and in the presence of antibiotics.
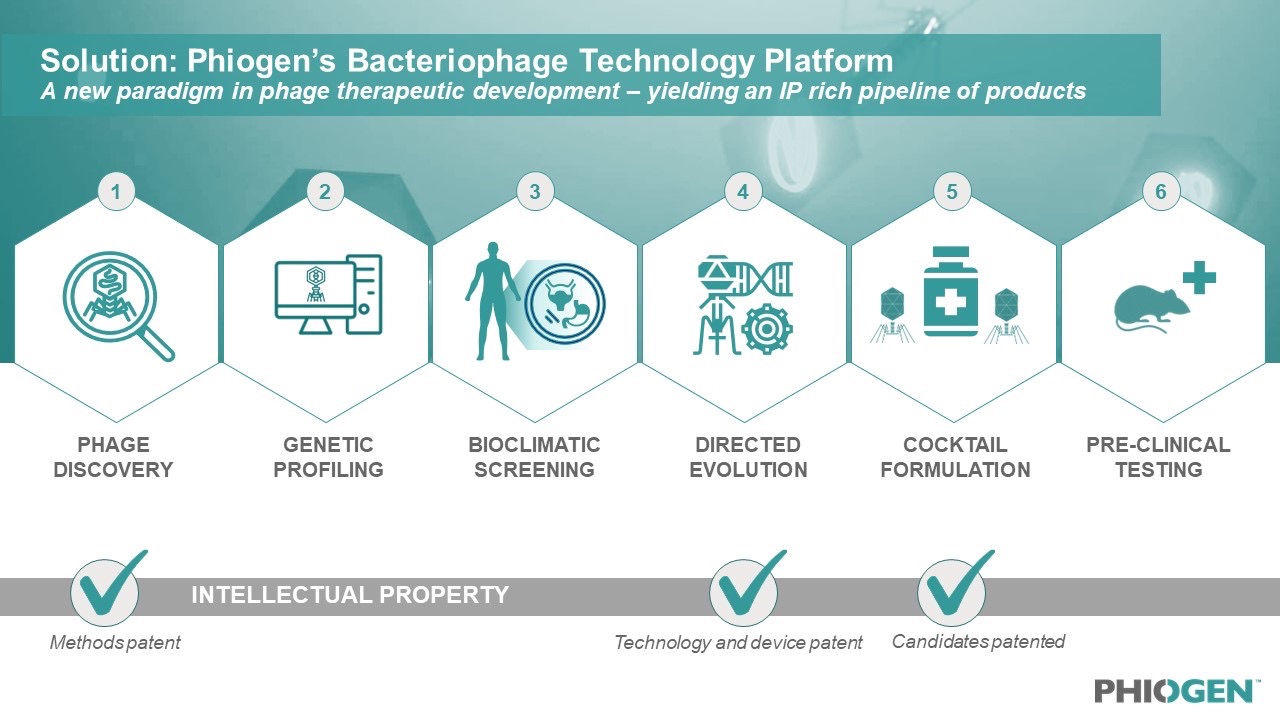
Figure 2: The world-first technology platform allows for high-throughput screening of phage candidates to be rigorously tested, validated, de-risked for any pathogen and formulated for any indication; including pathogens on the WHO’s high priority list.
During discovery, PHIOGEN uses a patent-pending technology that concentrates environmental samples without chemical treatment by over 10,000-fold in just a few hours. This allows scientists to continuously sample sources of rare phages that are both potent and novel which would otherwise be missed through traditional collection methods. The rarity of phages found through this process was recently validated after a hard-to-kill strain of bacteria was collected from a patient and failed to find any phage matches from a number of institutions across the US. With no other treatment options available, the patient’s strain was screened against the highly concentrated rare phage mixture which successfully resulted in several lytic phage hits to this once “untreatable” bacterial strain. Clinical isolates and phages identified from this method have yielded pairings that have been added to PHIOGEN’s development platform for further R&D and commercialization efforts.
After genetic profiling of the phages for safety and characterization, the company uses bioclimatic screening to confirm these rare phages for activity in physiological conditions that mimic the human infection microenvironment. This includes the use of colonoids, bladderoids, pulmonoids, human fluids such as blood and urine, and other simulated settings that mimic implants or devices. By screening phages in the environment that bacteria and infections naturally occur, PHIOGEN de-risks phages by selecting only those with unique properties that have already evolved to find their target and kill under similar conditions – essentially verifying efficacy in an “artificial patient”. Amanda Burkardt, PHIOGEN CEO, explained: “We like to think about formulating our cocktails with the end in mind and the best way to do that is to ensure your product works in the microenvironment – the human – you are asking it to perform in. As a result, we can make phages ‘druggable’ with broad coverage, breaking through the limitations of precision medicine and treating populations of patients versus on a per patient basis; one of the largest hurdles facing the business model of phage therapy.”
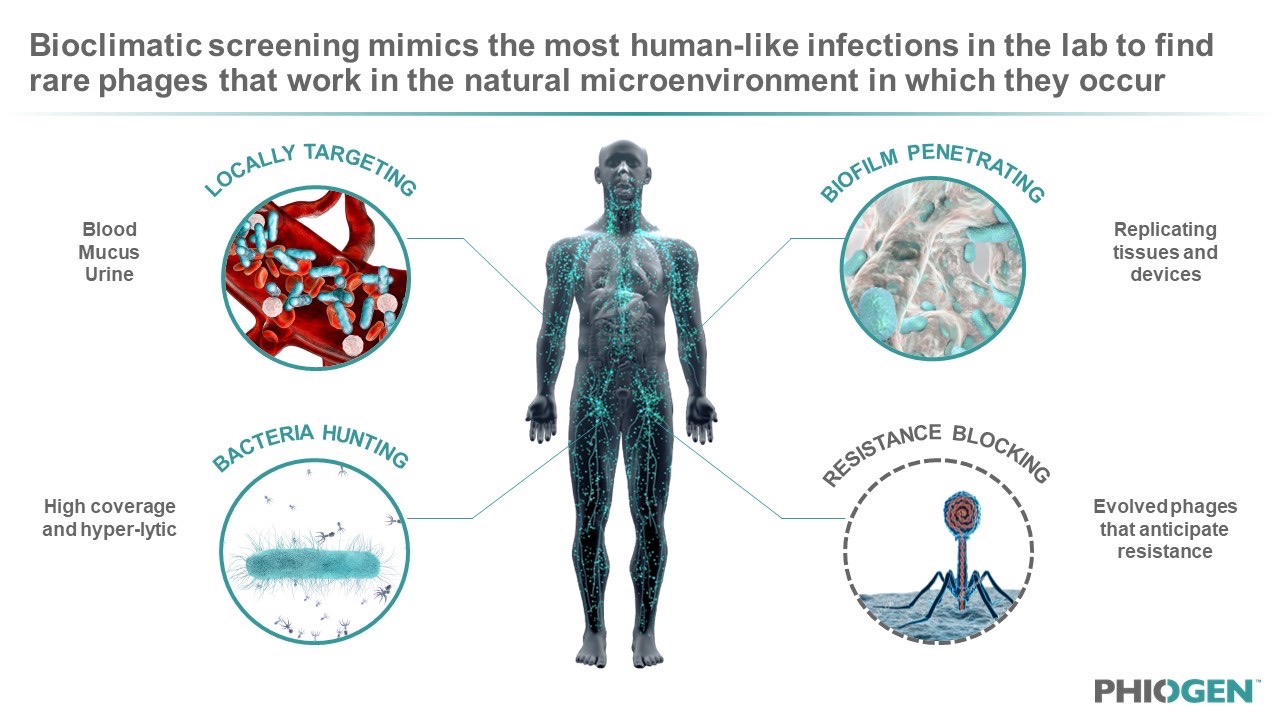
Figure 3: Bioclimatic screening allows for the modelling of phage-bacteria interactions in the body to find rare phages with superior anti-bacterial functions that normally get missed during the current standard of testing via LB or TSB plates and Agar.
The phages are then trained to adapt to resistant bacterial strains or acquire other functionalities that can impact therapeutic outcomes. This is done using another world-first proprietary technology developed by the group that cycles phages on resistor pathogens and then selects for an evolved phage variant that kills the antimicrobial resistant lineage. By populating cocktails with such phages, the pathogen’s ability to develop resistance is substantially decreased by essentially creating “anti-resistor” phages that are programmed to anticipate and overcome bacterial resistance. This results in a library of phages with special properties, each designed to address a critical part of the bacterial infection, enabling PHIOGEN to bring to market the best performing phages by creating a broad-acting therapeutic. The team has developed several cocktails which are patent-pending and have evolved anti-resistor phages for a number of pathogens listed as ESKAPE pathogens. “Phages are smart machines constructed through millions of years of evolutionary engineering to find their target under conditions that are imperfect,” said PHIOGEN scientist Dr. Anthony Maresso, who founded TAILOR labs and whose research team created the approach and technology for PHIOGEN.
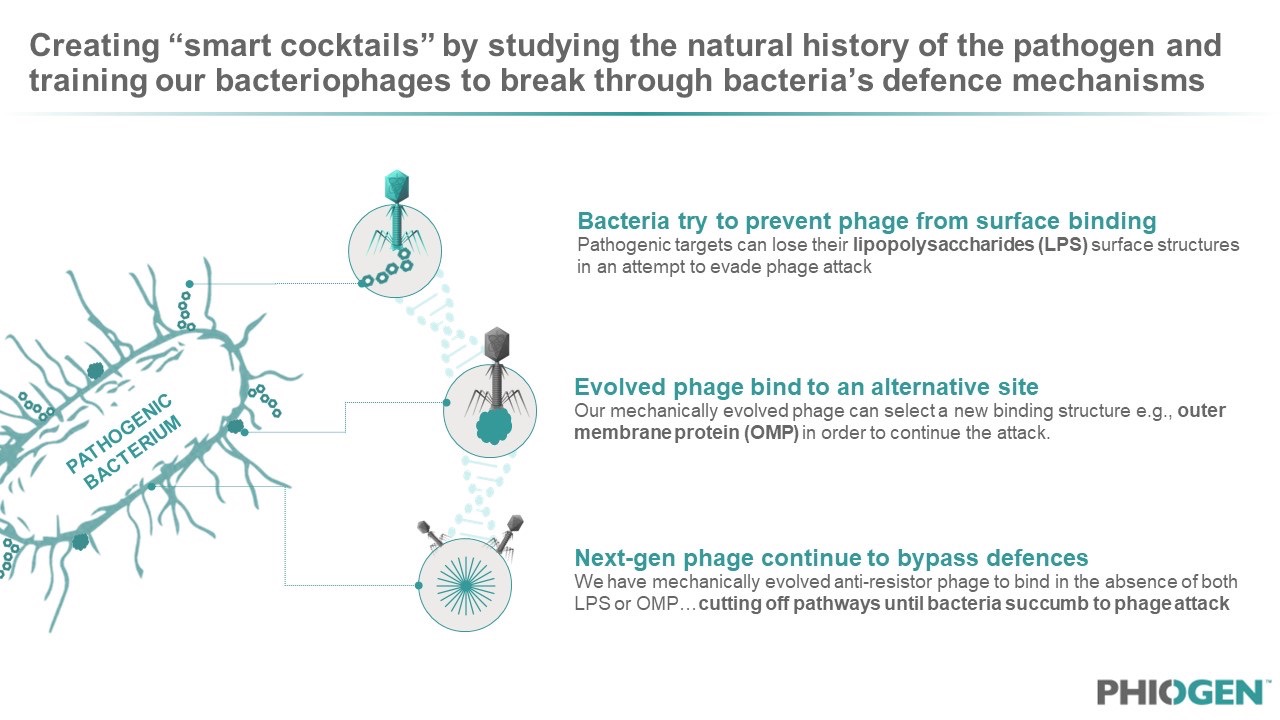
Figure 4: Patent protection has been secured around PHIOGEN’s “Directed Evolution” machine which allows for continuous phage-bacteria interactions that results in offspring phage which are evolved and programmed to be “anti-resistors”.
In the last stages of development, PHIOGEN focuses on formulations that are stable and has developed highly complex animal models to verify results seen in the bioclimatic screening stage. The whole technology platform allows products to be de-risked, reducing the need for unnecessary trials and improving clinical outcomes. The company has access to some of the U.S.’ leading expertise on phage manufacturing, safety testing, characterization, and readiness for regulatory assessment, which ensures the product meets all regulatory and safety criteria before entering trials.
Key to any modern therapeutic company is how its platform meets the changing medical needs of the future. Taking a quality over quantity approach, PHIOGEN harnesses the remarkable natural ability of phages that have already solved many of the challenges bacteria cause and carefully benchmark these qualities across accepted industry and regulatory standards of performance. By carefully selecting the most capable fighting phages and using directed evolution to unlock genetic superiority, PHIOGEN leverages a critical middle ground between engineered and completely natural phages to maximize function and adaptation. Through this method, PHIOGEN’s phages have already produced strong evidence of efficacy, with several patients in FDA-approved compassionate use cases showing positive outcomes.
“If I am an investor putting millions into a start-up and need the best science to maximize efficacy in a clinical trial, my best bet is on a process that has harnessed Earth’s most fit phages. The human body is not made of Luria Broth and Agar. Only by maximizing evolution’s biodiversity will we find the best acting medicines of tomorrow,” commented Dr. Maresso.
By utilizing this state-of-the-art technology platform, PHIOGEN can decrease costs associated with product development and reduce the occurrence of negative outcomes. Although the company has access to a library of hundreds of phages to cover all ESKAPE antibiotic resistant bacteria, the current focus is to target the largest market of bacterial infections, including urinary tract infections, bloodstream infections, and device-associated infections. The company believes not only are these markets economically sustainable, it is also where most patient suffering is observed, meaning any efficacious therapeutic will have a very meaningful impact on people’s lives.
The Path to the Future of Phages
PHIOGEN’s technology platform marks a significant breakthrough in the battle against AMR. By pioneering the evolution of natural bacteriophages that rival their genetically engineered counterparts, and selecting phages that are elite in the exact conditions of the target infection, the company’s approach holds tremendous promise by de-risking assets well before they are tested in pre-clinical and clinical trials.
The road ahead for PHIOGEN involves a robust R&D platform, clinical trials led by the nation’s top physicians, and regulatory approvals guided by experts in interfacing with the FDA, to bring transformative phage therapies to a wider population and solving the problem of scale. The urgency of the AMR crisis and the multi-faceted solutions that will be needed to address it mean cooperation from all stakeholders including investors, regulatory agencies, large pharma, industry, academic, and startups all playing a crucial role in accelerating the deployment of safe, effective, and scalable treatments.
The current clinical landscape accentuates the “better together” approach, where partnerships hold the key to effectively address this global crisis. By capitalizing on its breakthrough technology platform, decade of research, industry experts, and a creative vision moving forward, PHIOGEN is poised to shape the future of healthcare, offering hope in the face of what is now a desperate medical crisis. As is often stated in the phage field, “there’s a phage for that”. PHIOGEN is actively finding them and bring them to scale for patients that need them most.
“It ultimately distils down to this principle,” added Burkardt: “We want to be patient centric; to do good for the world and address this crisis of medicine with the very best science, technology, and brainpower in the field.”

Figure 5: PHIOGEN team and CEO, Amanda Burkardt, view imaging and screening machinery used to analyze safety and efficacy of phage products.
Further Readings
[1] Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis (The Lancet)
[2] Antimicrobial Resistance: Implications and Costs (Pubmed)
[3] National Infection & Death Estimates for Antimicrobial Resistance (CDC)