Pranav and Apurva Johri, the founders of Vitalis Phage Therapy, recently set up a new framework that allows patients in India to get phage susceptibility testing done locally. I interviewed Pranav and Apurva to learn more about what this milestone means, how they achieved it, and what the result/response has been so far.
Jessica: I’m so excited to hear about this key milestone for you and your organization, Vitalis Phage Therapy. Can you explain the milestone, and share what it means for patients in India looking for phage therapy options?
Pranav and Apurva: Yes, it is a major milestone indeed. We are excited to announce that we have been able to start diagnostic testing with phages in India, i.e. phage sensitivity testing of clinical bacterial isolates will happen locally. This cuts down the time, cost and other complexities associated with off-site diagnostic phage testing. It will not only accelerate the treatment process but also make it more accessible to people in India. Earlier, samples would have to be collected and shipped to the Eliava Institute in Georgia. This process was costly, involved additional paperwork and took about 3 weeks before a report could be generated. With this initiative, patients will be able to walk into the local pathology lab and get a culture report in 2 days and a phage susceptibility report in 5 days post sample submission.
J: That’s great news! Can you tell us a little bit more about how this idea was initiated and what it took to get it eventually implemented?
P&A: About 1.5 years ago, we set up an arrangement with the Eliava Phage Therapy Center of the Eliava Institute in Georgia to have distance/remote treatment done in India, establishing all the necessary framework and protocols. That really increased the accessibility for patients in India who wanted to access phage therapy but could not travel to Georgia due to either lack of financial resources or their medical condition not allowing them to travel. Slowly as the word started spreading about this new alternative treatment option for those patients with multi-drug resistant bacterial infections where traditional antibiotics were ineffective, the number of people approaching us kept increasing steadily. A few months in, we started getting contacted by people who were suffering from acute infections, some of them even hospitalized in the ICU. While the doctors treating them were willing to administer phage therapy, the turnaround time for diagnostics of about 20 days would be too long for their patients requiring immediate treatment. As the number of such cases grew, we soon realized that while having treatment locally available in India was the first step, we needed to bring testing to India as well to make treatment possible for people with urgent care needs.
Meanwhile, we started networking with the Indian health care community, presenting at various medical institutions and discussing the potential of phage therapy with doctors. During these discussions, we noticed an increasing number of Indian doctors acknowledged the rising issue of antimicrobial resistance and were open to exploring phage therapy options for cases where all other treatment options had failed. However, the enthusiasm dimmed when we explained the diagnostics process. The feedback we got was often centred around the need for a diagnostics process that was quick, comprehensive, and easily accessible, before the treatment option could be suggested to potential patients. So those two reasons were the trigger for us to decide that the next important step was to start local diagnostic phage sensitivity testing in India. Since then, it has been a long journey over 18 months, with our efforts finally culminating now with us reaching this goal.
J: Can you break down your approach to this challenging task? How did you get started and who did you have to contact first? At what stage did you have discussions with key players like the diagnostic centers, the regulatory authorities, and the Eliava Institute?
P&A: We started off by discussing this idea with the Eliava Institute first and explaining the need for local diagnostic phage testing in India for phage therapy to have a better reach and greater impact. In their long history, they had not had such an arrangement, where they had shared their phages with another lab for diagnostic testing purposes. So, we had to work with them to set up an extensive framework which addressed different aspects such as concerns about safety and security of the phages, testing protocols, storage requirements and so on. Since this step increased access to phage therapy, the Eliava Institute was supportive of this initiative and cooperated with us to make it happen. Once that framework was set in place, the next step was to choose a local diagnostic lab to partner with.
The most important factor that we looked for in our potential partner lab was whether their intentions aligned with the aim and spirit of our initiative. We needed a lab partner that aligned with our vision of enabling access to affordable phage diagnostics in India and would not try to commercially exploit this opportunity.
The next factor we were most concerned about was the capability of the lab. We needed a lab partner which understood and appreciated the concepts of phage biology and therapy from both microbiology and pathology perspectives. This would require an experienced and knowledgeable team of microbiologists, who would be willing to get trained on phage protocols and testing modalities. Once trained, we expected them to be able to set up procedures/protocols adhering to the provided guidelines to essentially replicate the whole testing process as done at the Eliava Institute and generate same quality results.
Another shortlisting criteria that we used to select a lab partner was that they should be able to get samples from all around the country. Our current partner lab, Dr Dangs Lab is based in New Delhi, but has courier partners in all major cities across India. Samples given to any of the courier partner locations are shipped overnight to the lab in Delhi. So far, we have tested 14 samples tested from 7 patients spread across 4 cities (New Delhi, Chennai, Bangalore and Raipur) and 80% of these samples were sensitive to Eliava’s standard phage preparations.
J: How much new training was required for the diagnostic partner’s lab staff? Is training a normal practice whenever they add new tests to their repertoire? How did the partner lab respond to the need for training?
P&A: The partner lab that we finally chose is an experienced lab having been around for many decades, but they had no previous experience with phages. Gladly, they were enthusiastic, right from the beginning, about the idea of undergoing necessary training for phage handling and testing. So, we arranged for a one-week training program at the Eliava Institute for the microbiologist who would be overseeing the local Indian diagnostic phage testing. They travelled to Georgia this February, luckily just before the Covid lockdown began, and spent a week there getting hands-on training on the entire process directly from the team that handles the diagnostic testing at Eliava. Once back in India, they were able to set up the testing process following the same protocols.
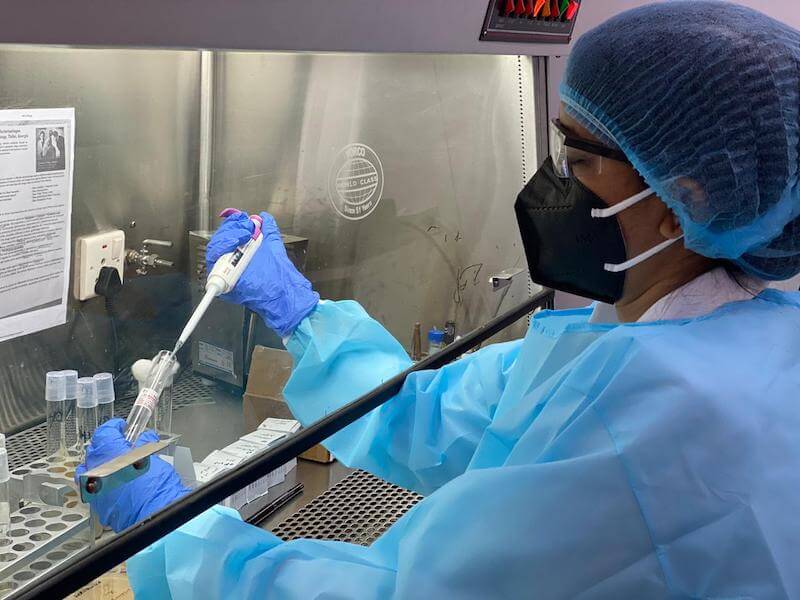
Dr Dangs Lab, a diagnostic lab in India, where phage susceptibility testing is now done for patients. Photo credit: Dr Devjani De.
J: Lucky indeed. So, how does it work for the diagnostic center? Do they keep a stock of all of the Eliava’s phages?
P&A: Eliava has six standard phage preparations as part of their first line of diagnostic testing. In case a sample tests resistant to all of first line phages, then a customized phage is prepared personalized for the patient. The local diagnostics lab here in India has testing quantities of each of the Eliava’s six standard page preparations. So far, most of the samples tested locally have shown sensitivity to these phage preparations. In the rest of the cases where the bacteria isolated do not show any sensitivity to these standard phage preparations, the bacterial samples have to be sent to Georgia for a customized phage to be prepared.
J: Say a patient gets their sensitivity results and they’re sensitive to one of the standard phage preparations. What happens after that?
P&A: The diagnostics lab generates a report which contains information such as extent of bacterial growth in the sample, sensitivity to different antibiotics and sensitivity to the phages tested. This report combined with the patient’s medical history report is then sent to the doctors at the Eliava. We then set up a telemedicine call for the patients and doctors to discuss various options for treatment plans and any concerns or questions from either side. Once the treatment plan is decided, the team at the Eliava Phage Therapy Center prepare a shipment of phages that is then sent to the patient along with the detailed treatment plan.
J: The telemedicine appointment is with the Georgian doctors, right? Do the local Indian doctors play any role in the treatment process?
P&A: Yes, the telemedicine appointment is with the Georgian doctors at Eliava, but the patients have the choice to involve their local Indian doctor in the telemedicine call. In cases where the patient may not be able to participate in the telemedicine call, due to hospitalization or personal preferences, the entire communication occurs directly between the doctors. Involving the local doctor helps, as they can monitor how the phages are administered, how the patient is responding to the phage administration, communicating that with the doctors at Eliava, and then tweaking the phage dosage accordingly.
J: It’s encouraging to hear that some Indian doctors are open to considering phage therapy as an option. How does the regulatory pathway work in India? Do these phages go through the compassionate use pathway that some other countries have been using?
P&A: Being a member country of the World Medical Association, India follows the Declaration of Helsinki, and allows compassionate use of imported medicines that may not be approved by regulators in India, as long as the product is approved for use in its country of origin by their regulatory body. Since phage therapy is approved by the regulatory authority in Georgia, it falls under the compassionate use framework. Just like compassionate use policies in different countries, documentation and applications to allow the import of the product need to come directly from the patient or a doctor treating the patient. Wherever necessary, we help patients with their applications and submitting the correct documents so they can get the approvals they need to allow the import of their phage medicines.
Diagnostic labs in India are accredited by the regulatory body NABL (National Accreditation Board for testing and calibration Laboratories). Accredited labs are allowed to conduct tests that have not been accredited yet. The phage diagnostic testing falls under this category, allowing our partner lab to run these tests under Indian regulations.
J: Let’s talk about the payment for the treatment. Does the patient end up paying for the treatment directly to Eliava? Can such treatments be covered by insurance?
P&A: The patient directly pays for the treatment to the Eliava Phage Therapy Center. Phage therapy is not covered by insurance anywhere in the world, as far as we know, so it is always the patient paying out of their own pocket.
While the local diagnostic testing is also paid for by the patient, we tried our best to minimize the costs while laying down the framework. We worked it out with our partner lab so that our culture and phage susceptibility tests would cost around the same as other normal culture and sensitivity tests they administer. To give you an idea, the culture and phage susceptibility test each costs around 850 INR, which is around $12, so it works out to be $25 per patient sample. If the samples have to be couriered from a different city, then the cost increases as courier costs get added to this.
J: Since you’ve created this new channel for phage susceptibility testing to be available in India, what kind of responses have you seen from local Indian doctors so far?
P&A: The response from the local doctors to our initiative has been quite positive. We had a few doctors who were waiting for a local diagnostics set-up as a prerequisite before they could discuss phage therapy as a potential treatment option. Now that the framework is set, they are willing to propose phage therapy as an option for those patients with drug resistant infections who have run out of antibiotic treatment options. We’ve also gotten the sense that having an established local diagnostic lab partner has increased their confidence in the entire protocol and treatment.
J: What was the hardest part of getting to this milestone?
P: I think conceiving the framework from scratch was the hardest part, because there is no precedent for this kind of set-up for us to follow. We first had to establish a framework which would accommodate all the prerequisites of the Eliava Institute concerning the safeguarding of the phages and the quality of the testing process, then find the right diagnostic lab partner and create a framework that would work for them. We also learnt a lot about regulations on phage therapy and how they differ in different countries through this exercise.
A: Another hard phase, not necessarily part of the process but post-completion, was the outbreak of SARS-CoV2. The COVID pandemic hit just as we were finishing setting up the entire framework and our local microbiologist was getting trained, and our planned launch got pushed back by at least six months.
J: Have people from other countries reached out to you with an interest in implementing a similar initiative in their own region or country? If so, are these phage researchers or members of the medical community, and how have these conversations been so far?
P&A: We have been contacted by people from various backgrounds: medical and non-medical researchers, scientists, clinicians, patients and sometimes even just curious citizens as the news of this initiative has spread. The inquires mostly focus on the process of setting up the framework, like where to start, what resources does one need to implement a similar structure in their region, how are people benefitting from it and so on. We think this interest really highlights the need for more frameworks to be in place to make phage therapy more accessible for different parts of the word.
J: How has your experience been working with the Eliava Institute on this initiative? Was it hard to convince them to get on board?
P: After an initial experience as a patient myself, we have been working with the Eliava Institute and Eliava Phage Therapy Center to advocate for phage therapy and to enable access to phage therapy for the last 3 years. Every step that we have proposed to increase access to phage therapy in India has been met with positive response and support from them. While distance treatment was something they had done before in various countries, setting up their phages out for diagnostic testing was unprecedented. So this initiative was fairly ground breaking for them too, and their support was necessary to get this started.
J: What role does Vitalis play in this initiative, and at what stage do you get involved? What are your plans for the future?
P&A: Our role has been to conceptualize and execute this entire framework to enable diagnostic phage sensitivity testing in India. Now that this has started, we plan to increase our outreach to clinicians and hospitals to discuss the potential of phage therapy. An increasing number of doctors are now aware of phage therapy as a treatment alternative to antibiotic resistant infections, and we hope that with the availability of local diagnostic testing, they will now start discussing this treatment option with their patients that are suffering from infections which do not respond to antibiotic treatments. One of our future plans is to document the cases and treatment experiences of patients in India in the form of case studies, which we plan to get published in scientific and medical journals. We hope that this step will increase the confidence of medical professionals in this form of treatment.
Further reading
This article was kindly transcribed and edited by Dr. Rohit Kongari, one of our Phage Directory volunteers. Thanks Rohit!
Thanks also to our new volunteers, Tolulope Oduselu and Atif Khan, for their work writing summaries and finding links for the What’s New, Jobs and Community sections this week!








