Over the years, as researchers have worked to bring phage therapy to fruition, a variety of barriers have stood in the way of adopting phage-based interventions as an alternative to conventional antibiotics. In clinical trials, phage formulations have been shown to quickly lose activity after being administered to test subjects, largely due to the innate structural fragility of these viruses. Additionally, beyond limitations in stability, the root issue of bacterial resistance remains a central limitation; just as bacteria develop resistance to conventional antibiotics, they can, and will, eventually develop resistance to the phages that they are being targeted by. It then stands to reason that switching to phage-based approaches merely changes the participants within the scheme of treatment-resistant bacterial infections, rather than overcoming the core issues themselves.
Origins of a solution
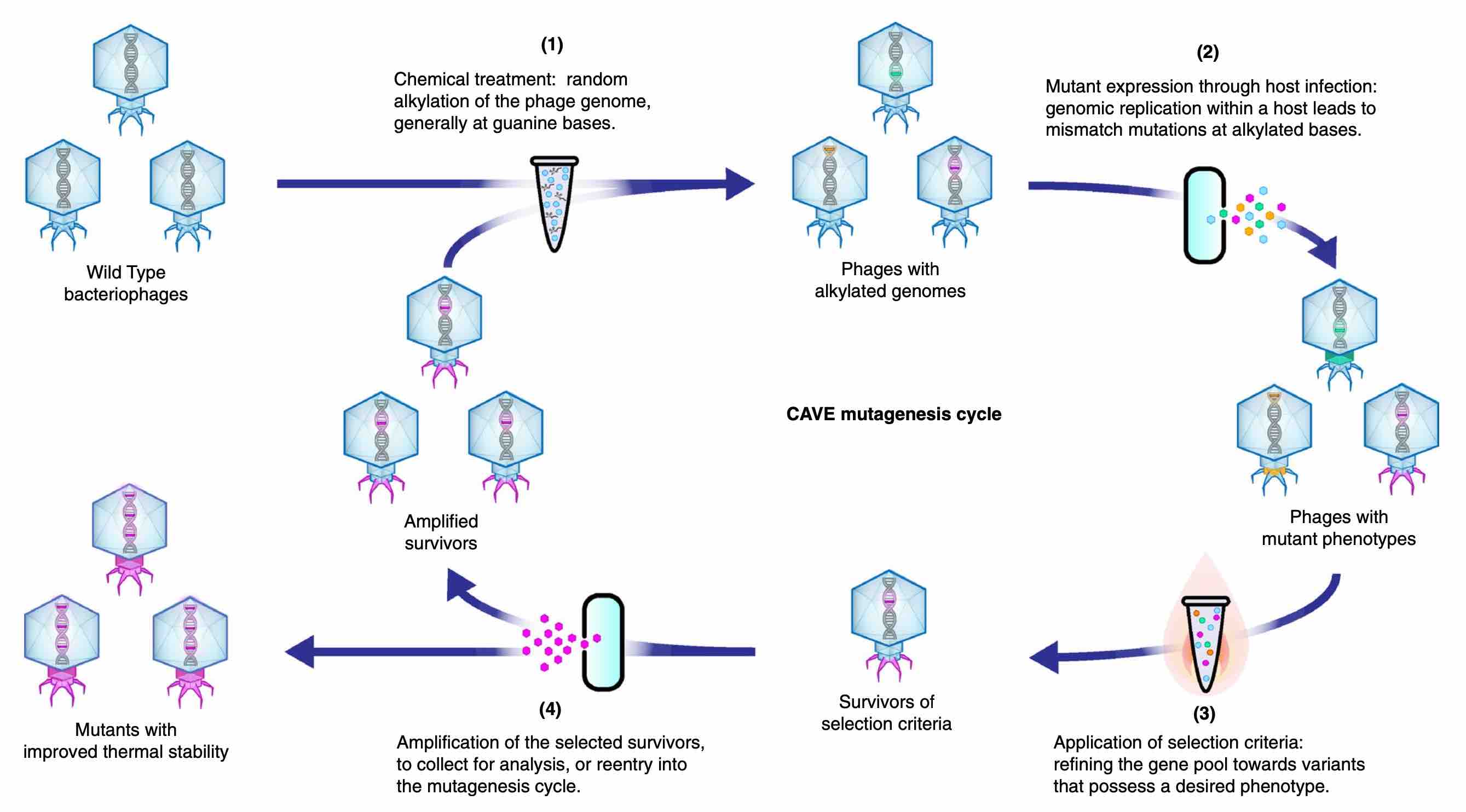
In our recent Scientific Reports article, Optimizing Bacteriophage Engineering Through an Accelerated Evolution Platform, we showcased a new methodology: Chemically Accelerated Viral Evolution (CAVE). This can be used to produce phages with enhanced thermal stability. The inspiration for this technique came about when our team faced issues involving the rapid loss of activity by phages that were being stored at even lenient temperatures (25-35 degrees Celsius). Our motivation for improving the survival rate of phages was threefold: we needed to find ways to extend the shelf life of phage-based products, promote their retention of activity at physiological temperatures during therapeutic applications, and ensure their stability during manufacturing processes. As we looked for ways to achieve this, we were drawn towards simple methods that had stood the test of time.
While scoping out various potential approaches, we stumbled upon some relatively old papers, from the 1950s and 60s, which described how alkylating agents like ethyl methanesulfonate (EMS) could induce random mutations in phages without inhibiting their activity. We decided to re-frame such observations in the context of a directed evolution scheme by developing an iterative protocol wherein each evolutionary cycle included a selection step so that the diversified phage gene pool would be gradually be guided towards the enrichment of desired traits.
Improving phage survival
In our initial efforts to improve the structural stability of our phages, we applied thermal-tolerance screenings as the selection steps, where mutant phages were incubated at high temperatures, only allowing variants with enhanced heat-resistance to survive. This method actually ended up being extremely effective in improving phage stability, and the innate simplicity of the platform suggested great versatility; the mutagenesis protocol could work on virtually any phage, and the selection steps could easily be modified in order to create phages with different sets of desired properties. Additionally, by studying the mutation patterns that arose over the course of evolution, we could gain great insight regarding the structural biology of phages. As we continued to characterize the properties of our newly produced phages, it became apparent that this simple method had provided an effective way to overcome one of the most significant limitations of phage therapy: the ability of phages to survive and retain their activity.
Changing host preferences
Since our initial experiments, we have refined high-throughput methods for producing evolved phages to meet a variety of needs. In addition to evolving thermally tolerant phages from a diverse range of families, we have used our technology to change host preferences from those of wild type phages. This technique has led us to develop new phages to target emergent strains of pathogenic bacteria in an efficient manner. This allows us to avoid the classic struggle of finding candidate phages in the wild that might possess activity against such hosts. One example of this application’s value was seen when we received samples of antibiotic-resistant Salmonella. Starting with wild type phages that initially displayed little or no lytic activity against these hosts, we were able to successfully evolve novel phages that could effectively target a majority of the provided strains. These results have revealed some of this technique’s most exciting potential, and we look forward to presenting them in greater detail in a forthcoming publication.
Conclusion
In the world’s efforts to develop antimicrobial agents, it has consistently been difficult to keep up with the rate at which bacteria develop resistance to the antimicrobial agents to which they are subjected. However, by having a rapid in vitro platform for enhancing phage characteristics, we have found a way to evolve phages in a manner that improves their therapeutic efficacy and expands their host ranges. With an ability to produce effective and diverse phages when needed, we finally have the necessary tools to keep up with this evolutionary arms race in a way that is aligned with the natural dynamics of phage-host systems. Ultimately, we see this technology as a powerful method for overcoming the barriers of phage therapy, and its effectiveness and flexibility provide great promise in actualizing the potential for phages to meet the needs of our modern world.