Unique properties of phages present challenges in production and purification. In this article, we will explore the key considerations in phage purification and the innovative techniques used to overcome these challenges. If impurities are not removed to the required extent, they can compromise the efficacy of the treatment and pose risks to patient safety.
Challenges in phage purification
Producing therapeutic-grade phages presents several challenges, particularly in achieving efficient and cost-effective separation of phages from the remains of bacterial cell, such as host cell proteins, host cell DNA, and endotoxins, especially from Gram-negative bacteria.
Traditional packed-bed columns for phage purification are prone to clogging and reduced flow rates when dealing with larger particles. Reducing endotoxin levels to meet medicinal standards often requires extra purification steps. This significantly prolongs the process, increases costs, and lowers recovery rates. Alternatively, dilution can be used to lower amount of endotoxins per dose, but dilution also reduces the concentration of phages as an active ingredient.
CIMmultus monolithic chromatography: an effective solution
The ultimate goal of the phage purification process is to achieve excellent purity and recovery rates, while maintaining a high phage titer and their functional viability. Phages are known to be large and labile particles, so the selection of the appropriate type of chromatographic column is critical. Monolithic columns are particularly well suited for this purpose due to their unique properties. Monolith chromatography is named for the structure of material inside the chromatography column, which is a single, continuous block (or “monolith”) of highly porous material with no dead-end pores, unlike packed bed chromatography (also known as resin chromatography) or membrane chromatography. CIMmultus monoliths are a special type of monolithic columns, typically made from methacrylate-based materials. The design of CIMmultus monolithic columns is optimized for reusable and highly efficient purification of large biomolecules, such as bacteriophages.
Potential for phage purification on CIMmultus monolithic columns was first described in study by Smrekar et al. in 2008, in which T4 Bacteriophage was bound to QA CIMmultus column. However, with the introduction of CIMmultus OH and CIMmultus PrimaS monolithic columns the process of phage purification has been significantly improved, as described in Rebula et all. in 2023.
CIMmultus monolith chromatography has been proven to successfully address many of these challenges, offering distinct advantages in terms of efficiency and scalability compared to other types of purification, including traditional purification (e.g. TFF, ultracentrifugation, organic solvent) or alternative type of chromatography (e.g. membrane chromatography or traditional packed bed columns based on resin chromatography). Opposed to other chromatography solutions, flow through monolithic columns is almost exclusively laminar, as shown in figure 1. Laminar flow is important for preventing the formation of eddies with turbulent shears that can damage delicate viral particles. Minimizing turbulent shears contributes to higher recovery of infective viruses throughout the purification process.
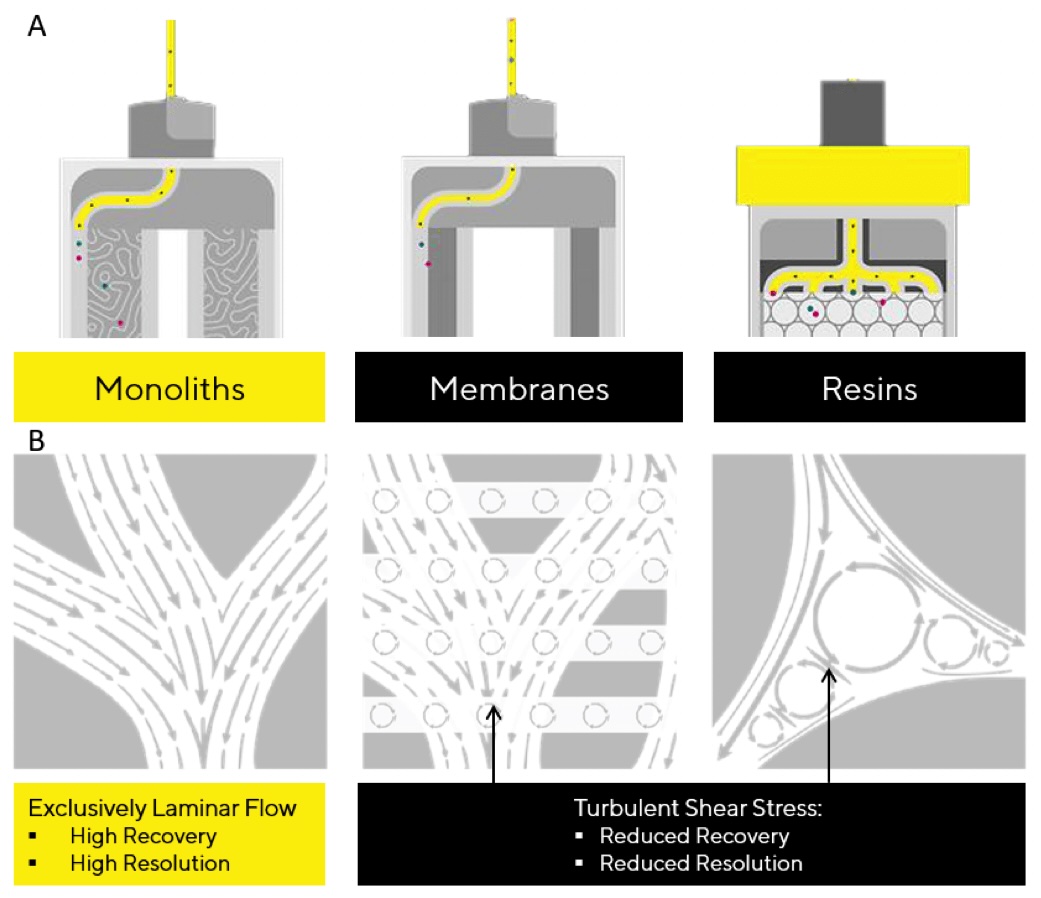
Figure 1: Cross sections of different chromatography media (A) and zoom ins (B) showing the flow strata. Turbulence occurs if the flow of fluid disrupts, forming eddies and vortices. This chaotic movement results in turbulent shear stress
These columns can process large biomolecules like phages rapidly and efficiently, allowing for high flow rates and low backpressure. With CIMmultus monolithic columns, there is no need for sample concentration via TFF before column loading. This significantly reduces process time, enabling the purification of a 2-liter lysate batch in just a few hours, saving valuable time without compromising the quality of the final drug product.
In addition, these columns are also highly reusable, capable of supporting multiple purification runs without significant performance loss. This reusability substantially reduces the cost per purification run, making the overall cost-effectiveness significantly better compared to traditional purification strategies.
Although the column capacity for a particular virus material depends on the purification steps applied prior to chromatography step, the superiority of CIMmultus monolithic columns compared to membranes and resins in terms of virus binding capacity was already demonstrated. Dynamic binding capacities up to 8.2E + 13 pfu/mL of monolith for different phage materials were previously reported. As CIMmultus monolithic columns are available in various sizes from 1 mL up to 40 L column sizes, this is sufficient for any volume of bacteriophage that needs to be purified.
Meeting rigorous purity standards for human therapy
When phage products are intended for treating bacterial infections in humans, the purity requirements are far more rigorous. If phages originate from a Gram-positive bacterial production system, filtration followed by a single chromatography step with a hydroxy (OH) ligand is typically sufficient to achieve industry-standard purity levels. This is because Gram-positive bacteria lack endotoxins, which are usually the most challenging impurities to remove from phage samples.
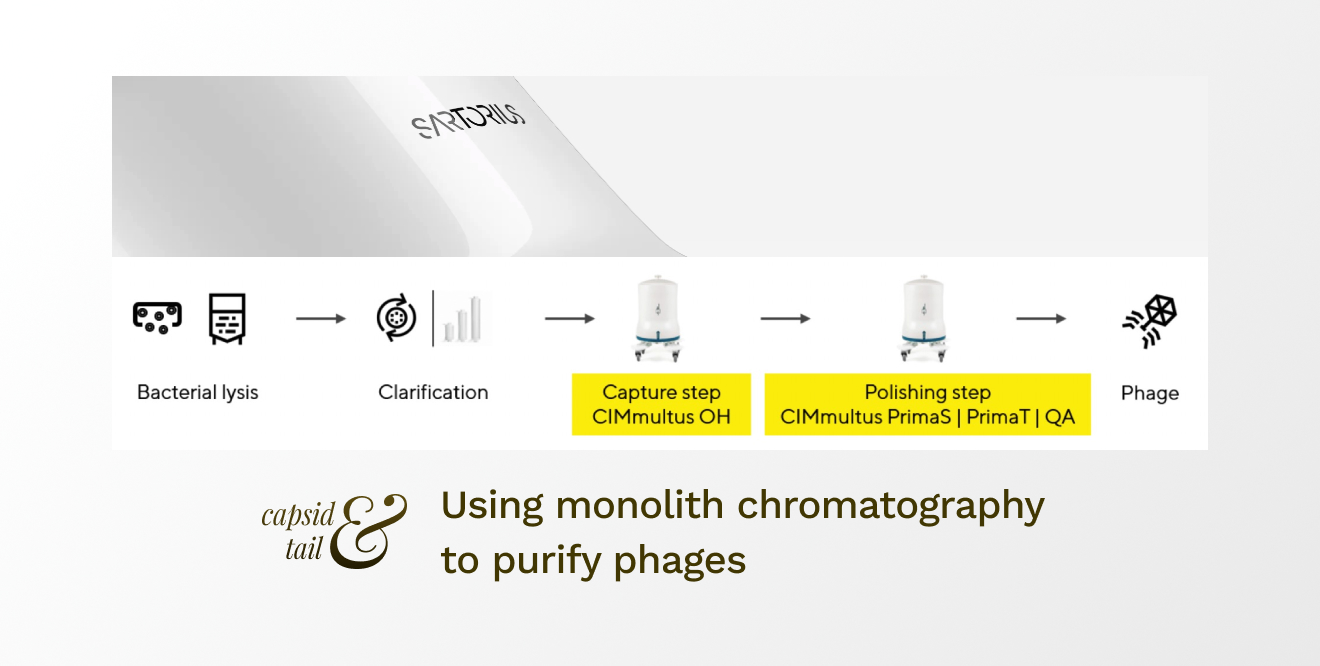
To reach stringent standards for human therapy if phages originate from a gram-negative bacteria, a two-step chromatography process is recommended (Figure 2).
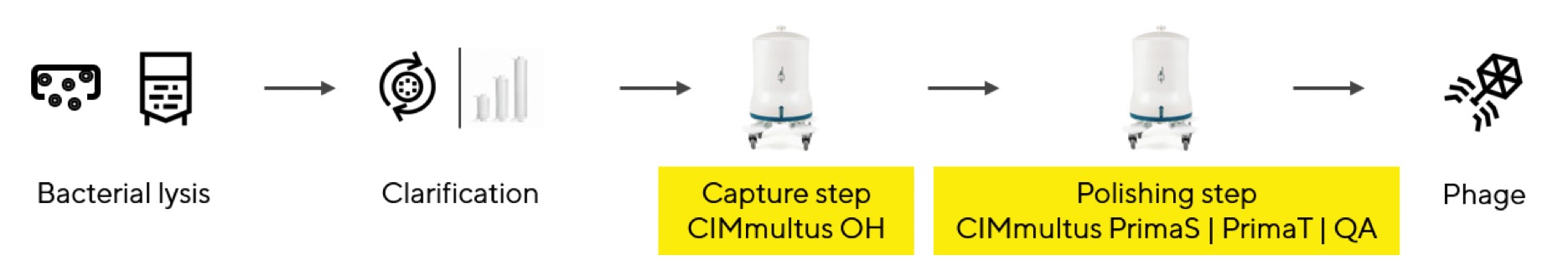
Figure 2: Two-step chromatographic purification of phage from gram-negative bacteria for human therapy
In the first step, a monolithic column with a hydroxy (OH) ligand is used to remove the majority of host cell impurities, achieving significant reductions in host cell proteins and DNA. The gentle interaction between the phages and the OH ligand ensures high recovery rates, typically maintaining 80-100% infectivity.
Subsequently, endotoxin removal can be performed with high efficiency using a weak anion exchange monolithic column with hydrogen bonding potential (PrimaS), achieving substantial reductions in endotoxins regardless of the type of phage. For instance, 5.8 mL loading sample with 5.29^6 EU for a phage with total 1.58^12 pfu and went down to 0.8 EU of 2 mL while still maintaining 1.04 ^12 pfu.
The elution buffer can be similar to PBS, potassium phosphate or SM buffer but with higher concentrations of salt to achieve the desired elution conditions. This allows the final formulation conditions to be achieved through simple dilution and the potential addition of essential stability additives to the elution phage fraction, thereby eliminating the need for a TFF step to prepare the phages for the final drug product.
Conclusion: the future of phage purification
In conclusion, CIMmultus monolithic columns represent a significant advancement in the field of phage purification, combining efficiency, scalability, and versatility. While the initial investment and optimization process may present challenges, the long-term benefits of using monolithic columns, particularly in terms of throughput and product integrity, make them a valuable and cost-efficient tool for both research and industrial applications.
Looking forward to working with you
Exploring new processes can be challenging for various reasons. This is why our process development Cornerstone team (email: [email protected]), is ready to develop purification process using CIMmultus monolithic columns with your phage of choice on a small scale and then transfer the process to your site. Additionally, our tech support team (email: [email protected]) is available to provide remote support, offering detailed guidelines, suggestions for process improvements, and updating you on our latest publications. We’re here to support you at every step, helping you navigate potential challenges and celebrating your success. Don’t hesitate to reach out—we’re always happy to help.
Further readings & links
L. Rebula, A. Raspor, M. Bavčar, A. Štrancar, M. Leskovec. CIM monolithic chromatography as a useful tool for endotoxin reduction and purification of bacteriophage particles supported with PAT analytics. J Chromatogr B Analyt Technol Biomed Life Sci. 2023 Feb 15;1217:123606. doi: https://doi.org/10.1016/j.jchromb.2023.123606
D. Vandenheuvel, S. Rombouts, E.M. Adriaenssens, Purification of Bacteriophages Using Anion-Exchange Chromatography, in: 2018: pp. 59–69. https://doi.org/10.1007/978-1-4939-7343-9_5
F. Smrekar, M. Ciringer, M. Peterka, A. Podgornik, A. ˇStrancar, Purification and concentration of bacteriophage T4 using monolithic chromatographic supports, J. Chromatogr. B. 861 (2008) 177–180, https://doi.org/10.1016/j.jchromb.2007.05.048
F. Smrekar, M. Ciringer, A. ˇStrancar, A. Podgornik, Characterisation of methacrylate monoliths for bacteriophage purification, J. Chromatogr. A. 1218 (2011) 2438–2444, https://doi.org/10.1016/j.chroma.2010.12.083
P. Kramberger, R.C. Honour, R.E. Herman, F. Smrekar, M. Peterka, Purification of the Staphylococcus aureus bacteriophages VDX-10 on methacrylate monoliths, J. Virol. Methods. 166 (2010) 60–64, https://doi.org/10.1016/j.jviromet.2010.02.020
P. Kramberger, L. Urbas, A. Štrancar. Downstream processing and chromatography based analytical methods for production of vaccines, gene therapy vectors, and bacteriophages. Hum Vaccin Immunother. 11(4) (2015) 1010-21. https://doi.org/10.1080/21645515.2015.1009817
E.M. Adriaenssens, S.M. Lehman, K. Vandersteegen, D. Vandenheuvel, D. L. Philippe, A. Cornelissen, M.R.J. Clokie, A.J. García, M. de Proft, M. Maes, R. Lavigne, CIM® monolithic anion-exchange chromatography as a useful alternative to CsCl gradient purification of bacteriophage particles, Virology. 434 (2012) 265–270, https://doi.org/10.1016/j.virol.2012.09.018
H.M. Oksanen, A. Domanska, D.H. Bamford, Monolithic ion exchange chromatographic methods for virus purification, Virology. 434 (2012) 271–277, https://doi.org/10.1016/j.virol.2012.09.019
K. Liu, Z. Wen, N. Li, W. Yang, L. Hu, J. Wang, Z. Yin, X. Dong, J. Li, Purification and concentration of mycobacteriophage D29 using monolithic chromatographic columns, J. Virol. Methods. 186 (2012) 7–13, https://doi.org/10.1016/j.jviromet.2012.07.016
Li, J., Zheng, H. & Leung, S.S.Y. Potential of bacteriophage therapy in managing Staphylococcus aureus infections during chemotherapy for lung cancer patients. Sci Rep 13, 9534 (2023). https://doi.org/10.1038/s41598-023-36749-2