Phage lysins have made exciting progress. In less than two decades they emerged from the initial preclinical proof-of-concept studies to the most advanced novel class of antibacterials in terms of safety, efficacy and feasibility. And they do not come too early, with a looming post-antibiotic era not far ahead of us. Some claim we are already there. The rapid action of lysins and the low probability to trigger resistance development are unusual but attractive properties to refuel the pipeline of antibacterials.
The most fascinating property of phage lysins is their modular structure and the potential to follow a click & play approach with lysin modules to modulate the lysin’s antibacterial properties and specificity. Phage lysins can in principal be selected and engineered against any pathogen. While the most advanced lysins under clinical evaluation are currently native lysins, evolution taught us that when using a protein outside its natural setting, there is plenty of room to re-evolve and optimize its properties to the prevailing conditions in the envisioned application, such as a bacterial infection. Therefore, I consider this click & play approach for lysins as the equivalent of the decade-long successful chemical engineering of natural antibiotics, which delivered us up to five generations of semi-synthetic cephalosporins and four generations of semi-synthetic fluoroquinolones. Lysins thus represent a truly broad class of antibacterials, and in my humble opinion, it is obvious that these days are only the infancy of lysins.
When we entered the field more than 15 years ago, we were courageous enough to move to the then-considered no-go zone of Gram-negative bacteria. Though, there were evident reasons to do so. The number of available antibiotics to treat infections of Gram-negatives was alarmingly low, and lower than the number for most Gram-positive pathogens. And apart from extensive biochemical characterisations of the lysins of the T-series E. coli phages, knowledge about lysins from Gram-negative infecting phages was generally limited. Not that we felt confident about our case. The long-hairy outer membrane popped up as a formidable brick wall. Most antibiotics are not able to cross this barrier, only those smaller than 600 Da do. But even the smallest lysins are roughly twenty-five times larger than this threshold. After some prospecting studies with chemical permeabilizers, the solution turned out to be relatively simple and surprisingly successful. We expanded the modularity principle that was proven so many times for lysins targeting Gram-positives. Specifically, we fused selected outer membrane permeabilizing peptides to the lysin and demonstrated that such a peptide can act as a drill bit, pulling the lysin through the outer membrane. The very thin peptidoglycan layer of Gram-negatives is then all but an obstacle. These engineered lysins are today known as Artilysins® and commercialized by Lysando.
As often happens in science, these joyful initial successes brought us quickly to the next hurdle. Not any outer membrane permeabilizing peptide fused to any lysin is successful. Often moderate antibacterial activities can be achieved, but the true killers are almost as rare as a needle in the haystack. In a follow-up study, we constructed 54 modular variants using diverse peptides, linkers and lysin domains. Pairwise comparisons taught us that each module matters and affects the eventual antibacterial activity. The outcome was exciting, but it took four years to construct and evaluate all these variants. Generating modular variants composed of three to eight building blocks was not straightforward at all. Yet, a practically infinite number of combinations can be considered as there are literally thousands of peptides that can be fused to thousands of lysins using thousands of different linkers. The lysins themselves can be also composed of a large diversity of cell wall binding and enzymatically active domains. It was frustrating to smell the potential, but not being fully able to grab it.
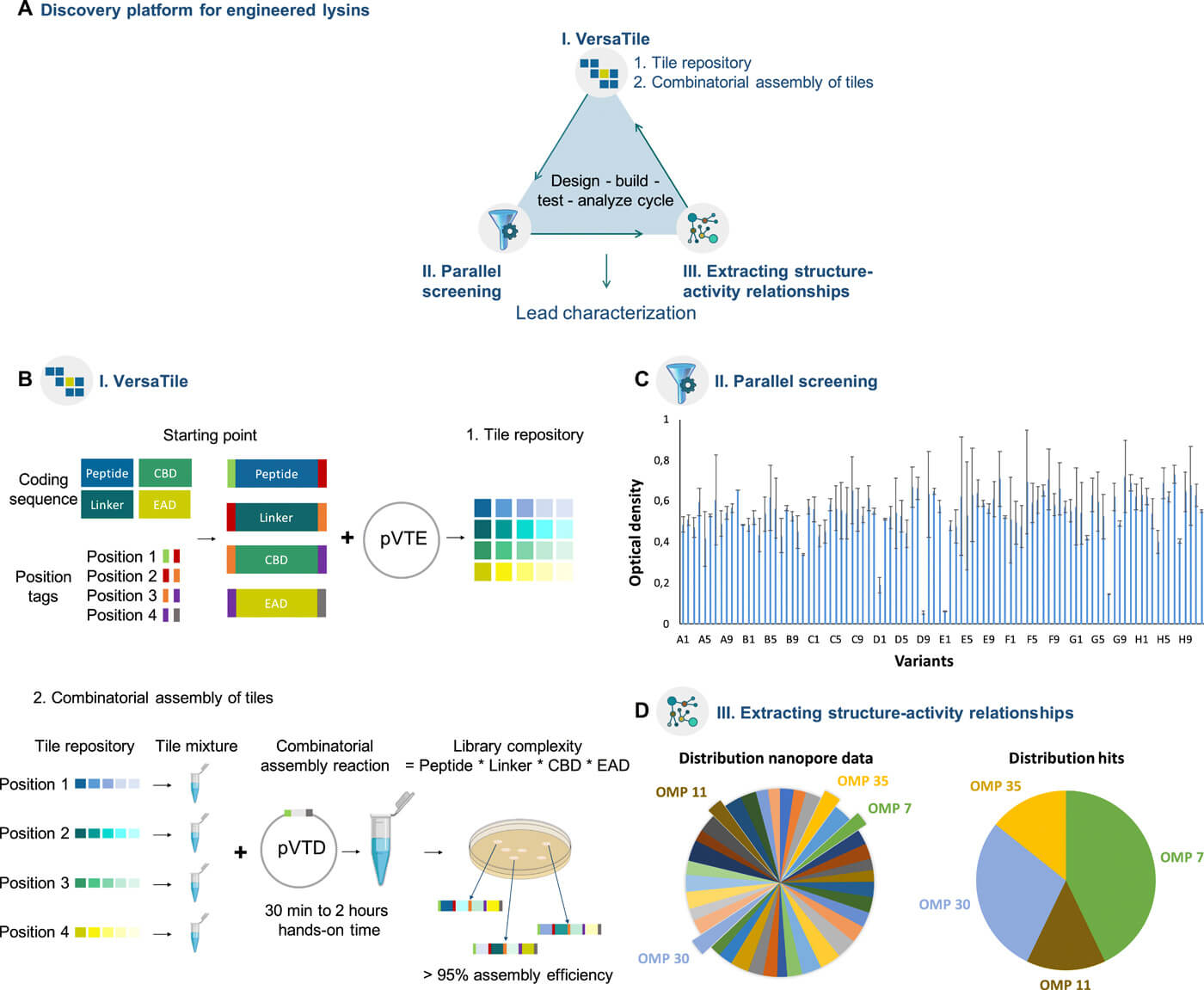
Frustration is often a good source for creativity. Turning back to basic gene technology, we designed and implemented a new method to eliminate this technical bottleneck, a method we coined VersaTile. VersaTile can be simply compared to Lego. You first make your collection of building blocks (‘tiles’), and then the endless joy of making new modular constructs can start. With VersaTile millions of modular lysins can now be constructed in a single step and a single day, ready for analysis. In our recently published proof-of-concept study, VersaTile was used to construct a library of 10,000 variants and a lead lysin killing Acinetobacter baumannii in human serum was identified by iterative screening.
We feel that the potential of VersaTile’s click & play approach is exciting, as the method is generic and can be applied to any modular lysin, and also any other modular protein. But as we learned before, tackling one hurdle means facing the next hurdle. We are now actively investigating novel approaches to deal with this high number of engineered lysins. Because we aspire that one day, lysins can be conveniently engineered against any pathogen under each condition, truly exploiting the most fascinating feature of lysins, their modularity.
Learn more
Rohit Kongari helped us produce this week’s article by helping us source and write the What’s New section. Thanks Rohit!!
Interested in becoming a Phage Directory volunteer?
Email [email protected].