I reached out to Dr Jessica Sacher through Dr Francesca Hodges who is lead of the Phage innovation network in the UK, to find out if it would be possible to visit the Phage Australia/Iredell Lab group and get some hands-on experience of how they produce phages as part of the STAMP clinical trial. Prof Jon Iredell (Director of Phage Australia and Centre for Infectious Diseases and Microbiology) and the team were very friendly and accommodating, and happy for me to come and visit and learn from them.
This visit was also very timely, as I am in the process of moving from a more phage discovery post-doc at the University of Liverpool (UOL), to working as a part of the Researcher in Residence initiative. This will involve still being a UOL researcher but working within CPI (Centre for Process Innovation) an industrial company, and working towards making phages on a large scale, to the high-quality standards (GMP or close to) to be able to use them to treat patients in the UK.
When I arrived at the Westmead institute (WIMR) Jessica firstly introduced to other members of the Phage Australia/Iredell team and showed me around the lab and the Institute. We then talked about each other’s phage research background. I was able to ask a lot of questions that I had from reading about the STAMP protocol and the phage production process. Jessica introduced me to Dr Stephanie Lynch and was able to pick her brains about the matching and biobanking side of the process. They also talked through how they split the different steps of the phage manufacturing process between the two of them and discussed a current patient that required phages and the difficulties that they’d been experiencing. This is really beneficial as I was able to see how some of the steps were troubleshooted.
Then Jessica showed me the pieces of equipment used in the actual production of the phages, these were the AKTA flux and AKTA pure and explained why and how they are used in a process. These are complex machines used to purify and detoxify the phage lysate. I was able to ask questions about each part to clearly understand and envision how it might be possible to upscale each technique, and learn about the equipment in more detail and why these machines are used to make the process more regulator friendly in a step towards being GMP.
After learning the theory of the phage production process and visually seeing the machines that are used on the first day, the following days I was able to trial some of the SOPs for different part for the process. Jessica and Stephanie have been working hard to make the SOP’s fool-proof so in the future anybody would be able to complete it without a phage background. While going through the SOP’s I was able to ask questions and provide suggestions to parts to add as I was the first from not in the lab to trial them. Due to my phage background, I was able to offer alternatives which may speed up the process in the future.

Figure 1 – AKTA flux for phage purification
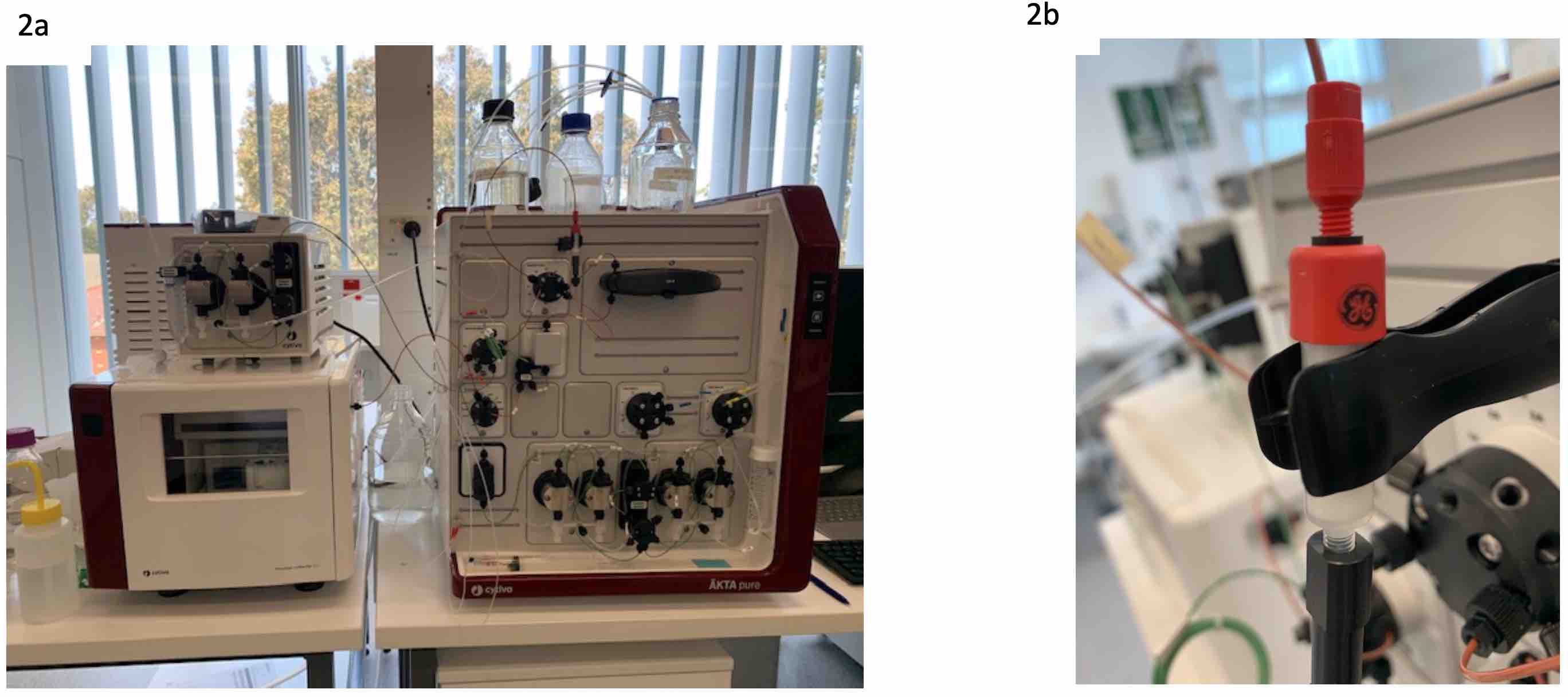
Figure 2. a) AKTA pure, again used in phage purification but the endotoxin removal stage of phage purification; b) the column used to remove the endotoxins from the phage preparation
I had the exciting opportunity to help with the QC stages for two phages that were being given to a patient this allowed me to understand all the reports and quality measurements that are required for the phage to be classed as safe and stable enough to be used in treatment. This stage is the part of the process that I found the most useful to take back to the UK as if we can prove that the phages are safe using end-point QC measures it may be possible to start treating patients before they are made to GMP standards. To note, the four QAQC reports need to be signed off by the HREC (Human Ethics) and Drug Committees at Westmead Hospital. The scientists and clinicians who signed off on the reports, namely Jon Iredell, Stephanie Lynch, Nouri Ben Zakour, and Jessica Sacher, are indemnified by the NSW Treasury Fund. Although I didn’t get to see the “behind the scenes” of the genomic pipeline, I am aware of its complexity.
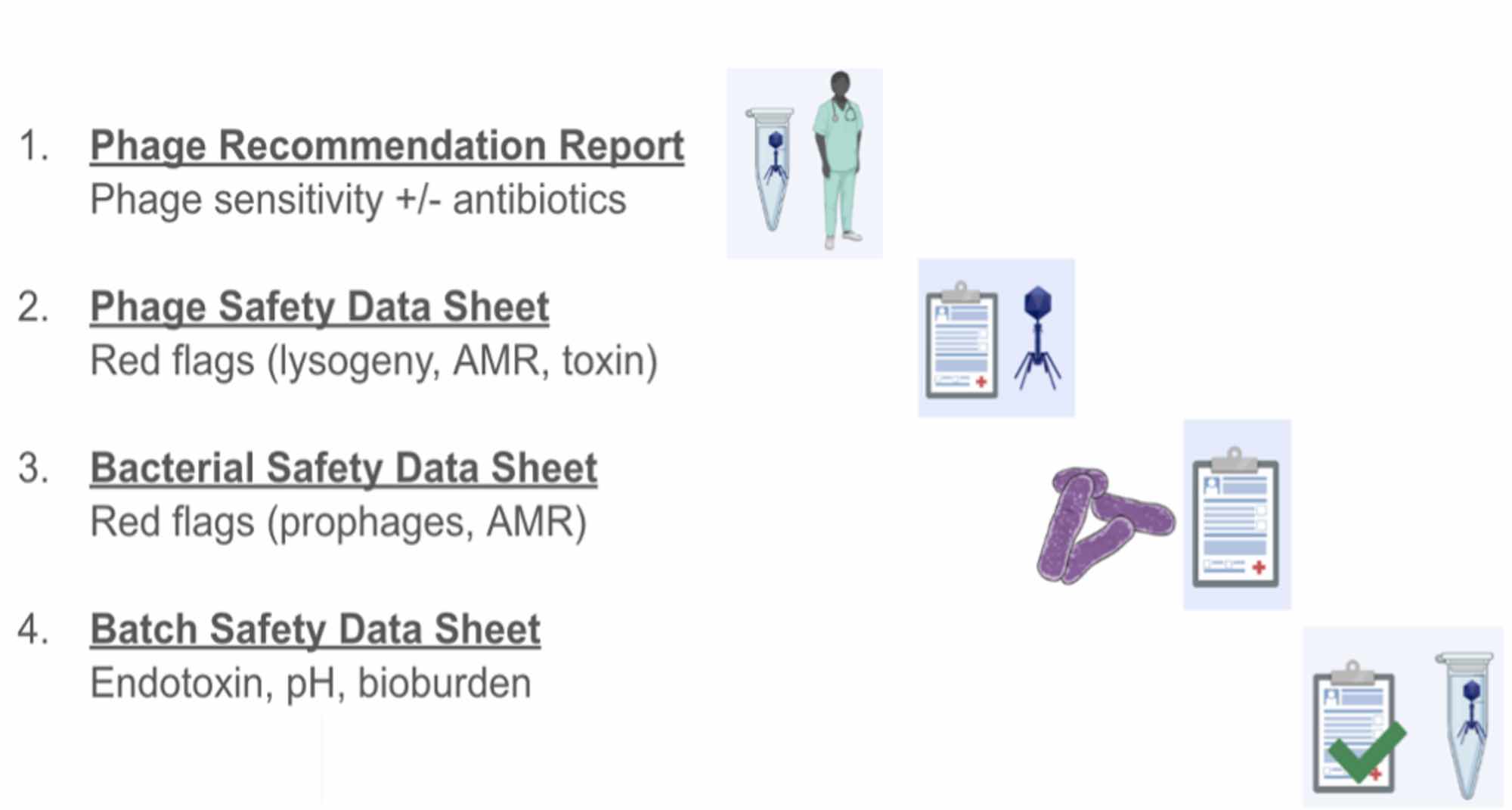
Figure 3. A summary of the documents that are submitted with every phage prep to demonstrate the safety of the contents, as well as the data backing up the phage choice.
One of the highlights of my visit was being able to sit in as an observer on the weekly clinical round meeting chaired by Prof Jon Iredell and Dr Holly Sinclair as this was completely new to me. This is where potential candidates are triaged and discussed. Getting to hear about the process leading to the next stage of phage therapy and finding out the wide range of alignments they are suffering from was eye-opening. As well as listening to Holly and other clinical colleagues (n>6) discuss the different complex situations of each patient while choosing the most appropriate order for them to receiver phage therapy. This was also my first experience of seeing lab scientists and bioinformatician including Jessica, Stephanie and Nouri inputting into a clinical meeting helping make clinical decisions on patients.
I thoroughly enjoyed my time at Phage Australia (headquartered at the Westmead Institute), the Phage Australia’s integrated operation has been going for over 6 years (Profs Jon Iredell & Ruby Lin), with funding injection of MRFF Frontiers in 2021 and is something to be highly respected with a small team of two main lab members on phage matching and production, it’s amazing what they have achieved so far. I feel like the knowledge I have gained and the skills I have learnt have been invaluable. As well as the truly insightful discussions I was able to have with fellow phage biologists, as things are done slightly differently between labs and countries, it was so interesting to hear different ways we have troubleshooted the same problem and came to the solution and even better to pick tips and ideas. I feel greatly enriched as a scientist by the whole experience.










