A team of Hungarian scientists created a global ‘superbug map’, paving the way for scalable phage therapy to combat antibiotic-resistant infections in hospitals
The fight against antibiotic-resistant infections has received a significant boost with the publication of the world’s first ‘superbug map’ for Acinetobacter baumannii, intended to aid the development of phage therapies.
Tracing A. baumannii around the world
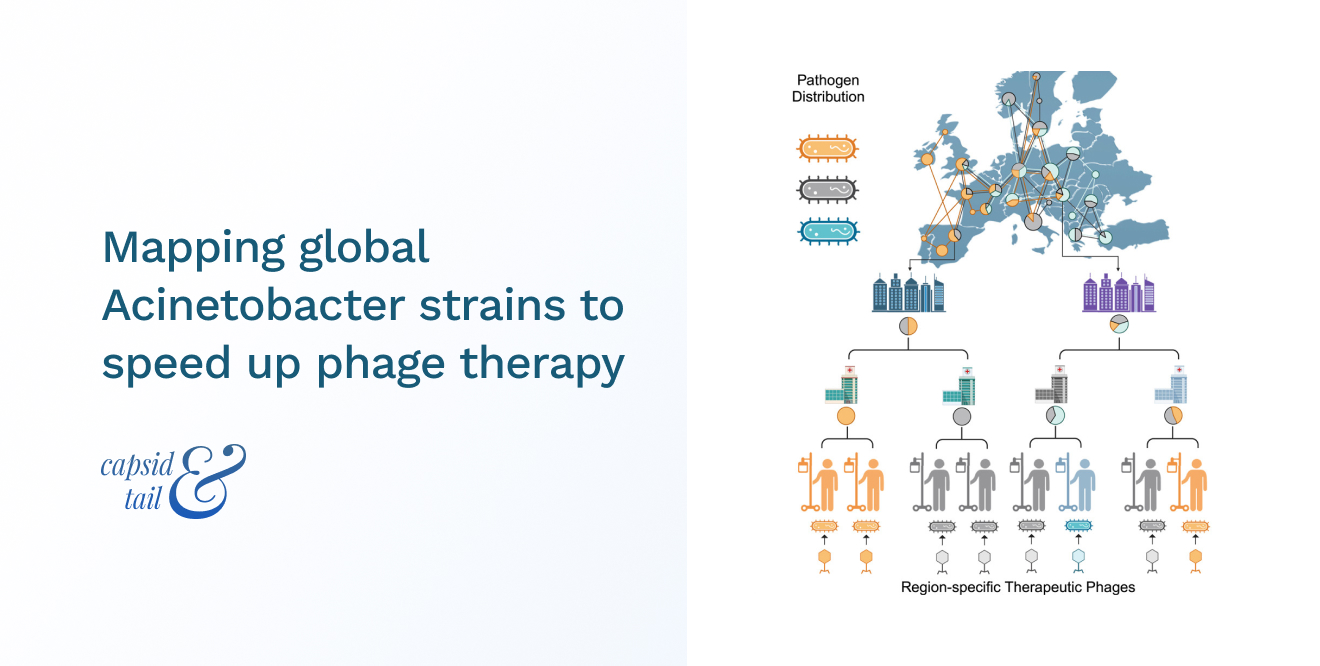
In a paper published in October in Cell, an international collaboration led by scientists at the HUN-REN Biological Research Centre in Szeged, Hungary, analysed the genomes of over 15,000 cases of Acinetobacter baumannii, tracing its global existence and spread.
The team identified the dominant strain types from around the world, discovering that the specific strains spread relatively slowly and remained dominant for approximately six years in a given country. As Acinetobacter phages are specific to strain types, this observation highlights the timeframe that hospitals may have to apply pre-emptively prepared phages for treatment as ‘off-the-shelf’ region-specific phage therapies that could address up to 80% of local infections.
“Currently many bacterial infections cannot be effectively treated and phages offer a promising alternative or complementary therapy to combat the rising number of hospital-acquired antibiotic-resistant infections,” said Balint Kintses, lead author of the paper and Group Leader at the Laboratory of Translational Microbiology at HUN-REN Biological Research Centre. “This is where our map offers a solution for phage therapy. By analysing how superbugs spread and dominate in hospitals, we can now prepare region-specific phage treatments in advance."
Helping clinicians move faster
To address the need for phage therapy in acute, AMR infections where patients die within days if a treatment cannot be found. The ‘superbug map’ is intended to enable the production of pre-emptive phage treatments based on the A. baumannii strains most likely to cause infections in each region. The same approach is used by scientists when selecting epitopes to target in the development of a new vaccine.
The hope is that, after turning these research findings into real-world treatments, healthcare providers will be able to significantly improve the management of infections that develop quickly and don’t respond to antibiotics.
“To reach this strategic goal, the superbug map will also help clinicians identify patients for phage therapy clinical trials, accelerating the validation and approval process for these treatments,” added Balint Kintses. “Researchers using the mapping framework will be able to access global data and share insights, contributing to a worldwide effort to combat antibiotic resistance through precision medicine approaches and bring phage treatments to the patients who need them most.”
“We are actively seeking collaborators willing to provide clinical isolates to help us evaluate the host specificity of phages on a truly global collection of isolates. If you’re interested in contributing, please feel free to send us an email,” said Balázs Papp, co-researcher and Group Leader at Lendület Laboratory of Computational Systems Biology at HUN-REN Biological Research Centre. “The ability to scale phage therapy is the key to making it a viable solution for hospitals worldwide.”
“This paper shows that an approach that directly addresses the diversity of phage targeting necessary for good therapeutic design,” said Prof Jonathan Iredell, Director of Phage Australia. “It also illustrates the open spirit of collaboration that is essential to success, and the generous provision of these novel therapies as a public good.”
Compiling the map
The map was compiled by conducting a large-scale analysis of over 15,000 genome sequences of A. baumannii from public databases from 85 countries across five continents to assess the geographical distribution of the strains.
Additionally, the team collected samples from 44 hospitals in five Eastern European countries from Covid-19 ventilated patients with secondary, antibiotic-resistant bacterial infections.
Using phylogeographical methods, the dominant bacterial strains in different regions were identified and their distribution tracked. This data was combined with high-throughput phage typing to understand which phages are effective against specific strains, enabling the development of targeted therapies.
Want to learn more?
For more information and to visualize the research, an animated video is available on the HUN-REN Szeged Biological Research Centre website.
Read the paper here: Koncz, M., Stirling, T., Mehdi, H. H., Méhi, O., Eszenyi, B., Asbóth, A., … & Kintses, B. (2024). Genomic surveillance as a scalable framework for precision phage therapy against antibiotic-resistant pathogens. Cell, 187(21), 5901-5918.