With COVID-19 giving viruses a bad rep, phage researchers have taken to twitter with the hashtag #NotAllViruses, and rightfully so! Bacteriophages (phages) are good viruses and are a promising alternative therapeutic against antibiotic-resistant bacterial infections. Their future use within the veterinary sector is promising, especially against infections such as canine pyoderma.
Antibiotic resistance within the veterinary sector is an important issue
Recent reports state 45% of bacterial isolates from companion animals are multidrug-resistant (MDR), able to survive administration of three or more different antibiotic classes!
One bacterium highlighted throughout this report, and the focus of our research, is Staphylococcus pseudintermedius, which causes a range of moderate to severe infections infection in dogs, where 78% of isolates are MDR. Of these infections, canine pyoderma is a significant concern, as it is the most commonly diagnosed skin infection within small veterinary practices.
With an increase in antibiotic resistance and a parallel decline in effective antibiotics, there is an urgent requirement for alternative therapeutics, with phage therapy explored as a novel treatment option in dogs.
Phages: a promising alternative in veterinary medicine
Did you know, the first successful trial of phage therapy in animals was conducted in 1919?
Fast forward 100 years and interest in phages has resurfaced due to the current antibiotic-resistance crisis. In 2010, Hawkins and colleagues conducted a successful phage therapy trial against Pseudomonas aeruginosa, an MDR bacterium that causes a chronic ear infection in dogs. Results showed the phage treatment reduced bacterial counts, which led to clinical improvement within 48 hours, and there we no signs of toxicity to the dogs.
With preliminary evidence of successful phage therapy in dogs, my research is focusing on phages and phage-derived products as a treatment for S. pseudintermedius infections in dogs.
The potential of phage therapy for Staphylococcus pseudintermedius
Phage therapy is an attractive option for the treatment of S. pseudintermedius infections in dogs, with a study by Moodley and colleagues already isolating four novel phages which preferentially lyse Methicillin-Resistant S. pseudintermedius (MRSP).
Additionally, as indicated by the TEM image below, we have isolated unique phages. They show narrow host ranges when tested individually, with an increase in lytic ability when combined in a phage cocktail, where they lyse 52% of S. pseudintermedius clinical isolates. Preliminary testing in vitro shows our phages significantly reduce bacterial counts within 20 minutes, with complete bacterial clearance by 2 hours.
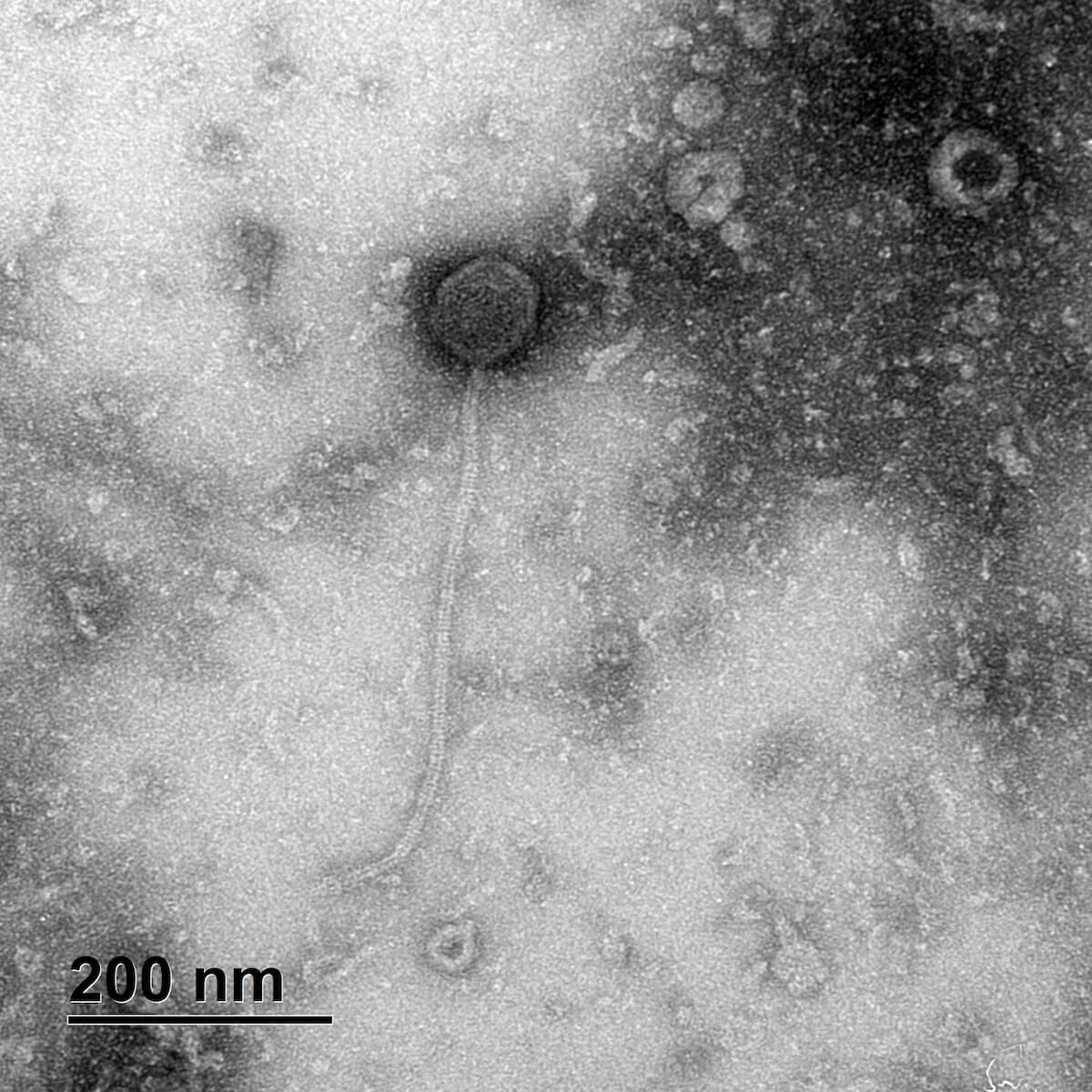
Transmission electron micrographs (TEM) image of phage S. pseud_22s.
Despite this promising data, genome characterisation of ours, and of previously published phages, show genes associated with lysogeny, which is unfavourable for phage therapy. Therefore, our research now explores the potential of endolysins against S. pseudintermedius.
Endolysins
Endolysins are enzymes synthesised by phages at the end of their lytic lifecycle to degrade the bacterial peptidoglycan to release phage virions. Junjappa and colleagues have shown that purified endolysin treatment results in moderate clearance of Staphylococcal infections with no allergic reactions observed in dogs. Therefore, we are interested in the potential use of endolysins for S. pseudintermedius specific infections in dogs.
We have selected 6 endolysins from S. pseudintermedius phages which are currently being manufactured. Upon synthesis, we aim to test the lytic activity of the purified endolysins on our clinical strains of S. pseudintermedius.
However, for phages or endolysins to progress to clinical trials in dogs, extensive trials in animal models are required.
Silkworm (Bombyx mori) as an alternative animal model for phage therapy trials
Currently, mice models are used for the safety and efficacy of phage therapy trials, however, recently, there has been extensive research focused on the development of alternative animal models.
Interestingly, Takemura-Uchiyama and colleagues have shown the results generated using the silkworm (Bombyx mori) model reflect results generated in mice models.
Using silkworms at the 5th instar stage, as displayed in the image below, we have shown that S. pseudintermedius can survive and replicate within the silkworm and is able to kill the silkworm in a dose-dependent manner. We have also shown that injection of our phage preparations individually shows no adverse side effects to the silkworms and is, therefore, a safe treatment option moving forward and will progress to upcoming trials in dogs.

Silkworm at 5th instar stage, used for phage therapy trials.
The future of phage therapy
Multi-drug resistant S. pseudintermedius is a significant threat within both veterinary and human health. The use of phages and endolysins, as well as the development of an alternative animal model, represents a significant step forward, and will set the groundwork for the progression of phage therapy into a clinical setting for the treatment of infections in dogs caused by S. pseudintermedius.
Rohit Kongari helped us produce this week’s article by helping us source and write the What’s New and Jobs sections. Thanks Rohit!!
Interested in becoming a Phage Directory volunteer?
Email [email protected].









