This week I’m excited to share how I’ve been using our new AKTA Flux system to purify phages for patients.
Though I’m all for automation in theory, this was an area I hesitated to dive into, as it seemed like an entirely different field… the words ‘bioprocessing’ and ‘biomanufacturing’ were not really in my lexicon.
But somehow, over the course of a few months, what used to feel overwhelming has actually become fun! I finally understand why the phage field needs a system like this, and what it can do for our team. Now I’m the one throwing around a bunch of words like ‘diafiltration’ and ‘transmembrane pressure’ in my regular speech, even though I’m a phage microbiologist and not an engineer or a ‘production chemist’.
How did this happen? Let’s dive in. (Maybe I can convince you to join me!)
⚠️ Caveat: I am a complete beginner to all this. This is my adventure delving into all this for the first time starting in March of this year. Please don’t take my words as absolute… I’m sure I’ve only got a surface level understanding, and I’m learning every day. But this is how I currently understand the AKTA Flux S, tangential flow filtration, and how I’m using it and thinking about it for phage purification.
The need to make pure phages
At Phage Australia, we really need pure phages. Patients are waiting. I know many of you are in the same boat.
As you’ve maybe read in my previous posts, I’ve been using spin columns and octanol (mainly following Bonilla et al.’s Phage on Tap method) to purify most of our phages over the last year.
In parallel, others on our team have been hard at work making it possible for us to graduate up to the next level, transitioning to using phage purification equipment we can automate, scale up to larger volumes, and most importantly, standardize and trust.
Big picture, this is all part of our plan to gradually transition toward GMP phage production. Although we’re still in a GMP-exempt window right now, to continue producing phages for patients like we’re doing, we need to keep earning the trust from our regulators, and that means making progress in a GMP-certifiable direction.
Apparently, Cytiva’s AKTA Flux system is one of the ways pharma, viral vector manufacturing, etc, are purifying their biologics.
And that’s about all I knew about these machines until very recently.
First, a few shoutouts
- Ali Khalid on our team has been developing the AKTA Flux methods I’ll be talking about below for the past year, by working with an awesome core facility here in Sydney (shoutout to Hélène Lebhar and Chris Marquis at the RPF at UNSW). Ali is always my first call when I have issues.
- Ruby Lin on our team has been pulling the strings for multiple years (!) behind the scenes to get us our AKTA machines — from getting the funding to figuring out what we need, to getting the machines to our benchtop — without her work, we’d be nowhere!
Serendipity: the old method fails right after our new machines arrive
It was exciting when the machines arrived. But it felt hugely daunting to get started. I decided I would start using them when I ‘had time’.
But then, soon after they arrived, our other method of phage-making failed. And a patient really needed those phages.
I was faced with a decision. Find a way to troubleshoot our old methods? Or bite the bullet and learn how to use the new machines?
Going forth into the unknown
I decided to go forth and try to get started on the AKTA Flux. It had to happen eventually — why not now? (Also, I do have a weakness for shiny new robots, and I generally enjoy diving into new experimental adventures once I decide to start.)
Luckily, the training I got from Cytiva was really great — shoutout to Christina Cahir, whose teaching style is generally ‘Go ahead. Try that if you want to. See what happens. Oh yeah, a leak. Yeah that’ll happen. What are you gonna do about it?’ [sits zen-like and watches me fumble around until I remember about the emergency off-switch]. This worked for me, and instilled just the right amount of confidence to get started.
Acronym insanity, panic
Luckily for me, I wasn’t coming in completely blind. But it felt that way. I’d been hearing Ali talk every few weeks about his AKTA method development for about a year. But it all sounded like gibberish to me. Not his fault… it’s just a lot.
First of all, there’s multiple types of AKTA machines, and they all do different things. Each one can connect to different columns and cartridges and filters. And of course, talking about any of this means a whole slew of new acronyms and words; things like Flux S, Flux 6, AKTA Pure. Anion exchange, TFF, Diafiltration, Buffer exchange. CFF. Tangential flow filtration. Cross flow filtration. Chromatography. Affinity column, polishing, water flux. Midgee Hoop. Hollow fibre.
So I’d heard the words, I’d even googled them… but it hadn’t stuck.
This post is my attempt at answering my own super basic questions (and the questions I’ve been getting from others in the phage field who’ve been asking). I’m aiming for a nice little primer to help ease you in, if you’re completely new to it, and curious what you might be getting into.
What is an AKTA?
First things first — ‘an akta’ is not one thing. AKTA is a product line; there are many models under the AKTA brand. Cytiva is the company who sells this product line. The AKTA Flux S is one of the models, and that’s the one I’ve been using. It is used for something called tangential flow filtration.
What is tangential flow filtration?
Tangential flow filtration (TFF) is sort of like sideways filtration. Imagine you’re filtering something, but instead of the fluid hitting the filter head-on, imagine the fluid flowing through a tube. Imagine that tube is itself a filter (it has holes in it). This first tube sits inside a second, larger tube, which doesn’t have holes in it. So as the liquid flows through the inner tube, it’s being filtered — the small particles that can fit through the holes leak out the sides of the filter-tube, along with some of the liquid. Most of the liquid stays inside the inner tube though, and it continues to recirculate through it (as long as you leave the thing running).
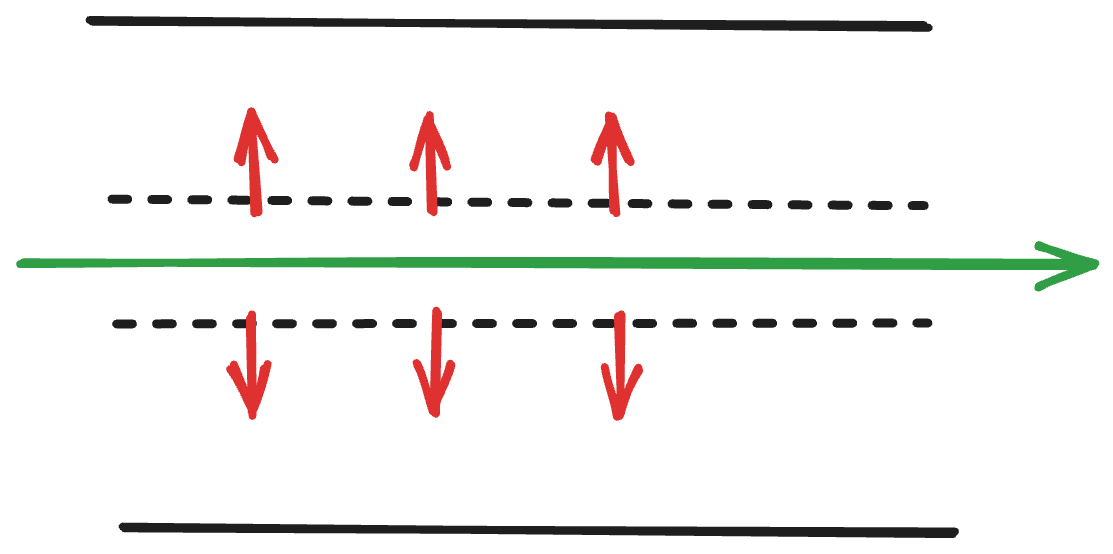
Fig 1. Here’s a zoomed in version of how I imagine TFF, where the green arrow is the phage lysate flowing through the porous inner tube (dotted line). The red shows what filters out (what you don’t want).
Why not just do regular filtration?
Regular filtration (flow hits the filter head-on) clogs the filter more easily (we’ve all probably been there, sweating through trying to push liquid through a syringe filter). Tangential flow filtration apparently doesn’t clog it as easily — the filter is distributed over way more surface area, and the flow helps dislodge the clogged stuff as it flows.
But I’ve heard it called cross-flow filtration and diafiltration…
Yeah, me too. They’re all basically the same. Tangential flow filtration (aka TFF). Cross-flow filtration (CFF). Diafiltration. People just seem to use these words interchangeably as far as I can tell.
Why is the AKTA Flux useful for phage work?
Many reasons!
- It lets you clean your phage lysate (yellow→clear).
- It lets you concentrate your phage lysate (1L? 10L?→ 50 mL; you get to decide).
- It lets you show exactly what you did, because it collects its own data on what the parameters were (like flow rate, pressure, etc, whether it was run according to protocol) as it runs (regulators will want to see).
- It helps with reproducibility and predictability (you’re telling a machine to do something instead of a person; less room for interpretation).
- It lets you set it and (mostly) forget it, like a dishwasher. (Contrast that to our previous spin column process, which would involve 30-40 small spins, and would take 4-6 h). (This would be the equivalent of turning on your dishwasher, setting the timer for 3 minutes, letting it come to a stop, opening it, taking out the dishes and checking if they’re clean, adding some water, putting the dishes back, and setting it to start all over again for another few minutes. All day. A dishwasher is so much better!)
How does the AKTA Flux work?
It’s essentially a computer (controlled by a touch screen) that controls the flow of fluid across a filter (Fig. 2). You buy the filter you want, and connect it to the tubes, and add your phage to the tank.

Fig. 2. Here’s the AKTA Flux S, and the main things you need to care about. It’s pretty simple! Set up your filter of choice on the right, add your phage to the tank on the left, set your parameters, and start the pumps.
The filter we like to use is called the Midgee Hoop. It’s a ‘hollow’ tangential flow filter like I drew in Fig. 1. above, and it happens to be circular (it’s a long tube, arranged in a loop, for convenience) (Fig. 3). There’s a tube that comes off the side that catches everything in the ‘outer tube’ — everything you don’t want. That goes to a waste bottle (which is called the ‘permeate’). You want a pore size that’s too small for your phages to fit through — we’ve found that 100 kDa seems to work for most phages.
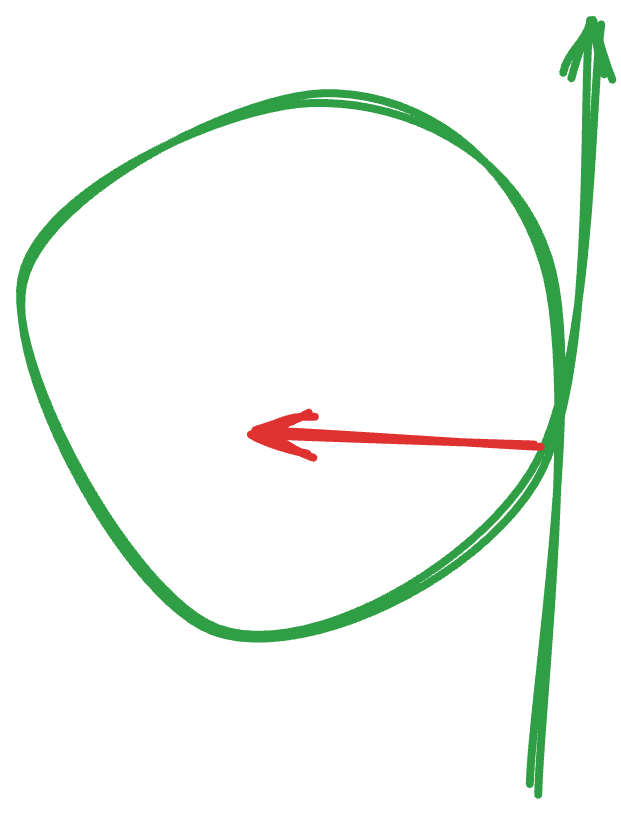
Fig. 3. Here’s my rudimentary drawing of the Midgee Hoop. The green is the flow path of the phage. The red is what comes off the filter, going out to waste.
When you start the pump, it starts pushing your phage from the tank through the tubes and through the filter. Most of the fluid does NOT come out as waste; only a small percent of it does. Most of it recirculates back into the tank, and the whole point is, you can recirculate your phage through the filter however many times you want (topping up the tank with clean buffer at various intervals), until your phage is clean enough for your liking.
Once it’s clean (you should see the tank liquid become colourless), or whenever you’re ready, you stop the pump and harvest your clean phage (along with whatever else was too big to pass through the filter). Then you clean the machine to get rid of all the phages (we use hot sodium hydroxide and it seems to work for most phages), test it to ensure it’s truly clean/no phages remain, and then it’s ready to be used again for another phage.
Controlling it
There’s a lot you can control about the process. This can easily get overwhelming, but you really don’t need to worry too much. The flow rate and the pressure are the main ones. You have a touch screen which lets you control the flow rate (in mL/min, ie. how fast is the liquid flowing?), and shows you the readings. You have a physical knob for controlling the pressure (’transmembrane pressure’, or TMP, is the main one that matters; too high and you may destroy your filter; too low and nothing will really pass through it).
You have a giant STOP button on the screen in case anything weird happens, like something starts leaking, or the pressure suddenly skyrockets — you just stop everything. No drama.
There is also a whole area called ‘Alarms’ which is fun to play with and helpful for letting you walk away without worries (again, can get overwhelming, but you don’t have to use it until you’re comfortable with the basics). For example, you can set the machine to stop or beep at certain tank volumes (oh look, it’s down to 50mL, that’s as concentrated as I need it to be — time to stop and add more buffer!). Or at certain pressures (uh oh, it’s crept up to 0.45 Bar, but I want it at 0.4, better adjust it back down).
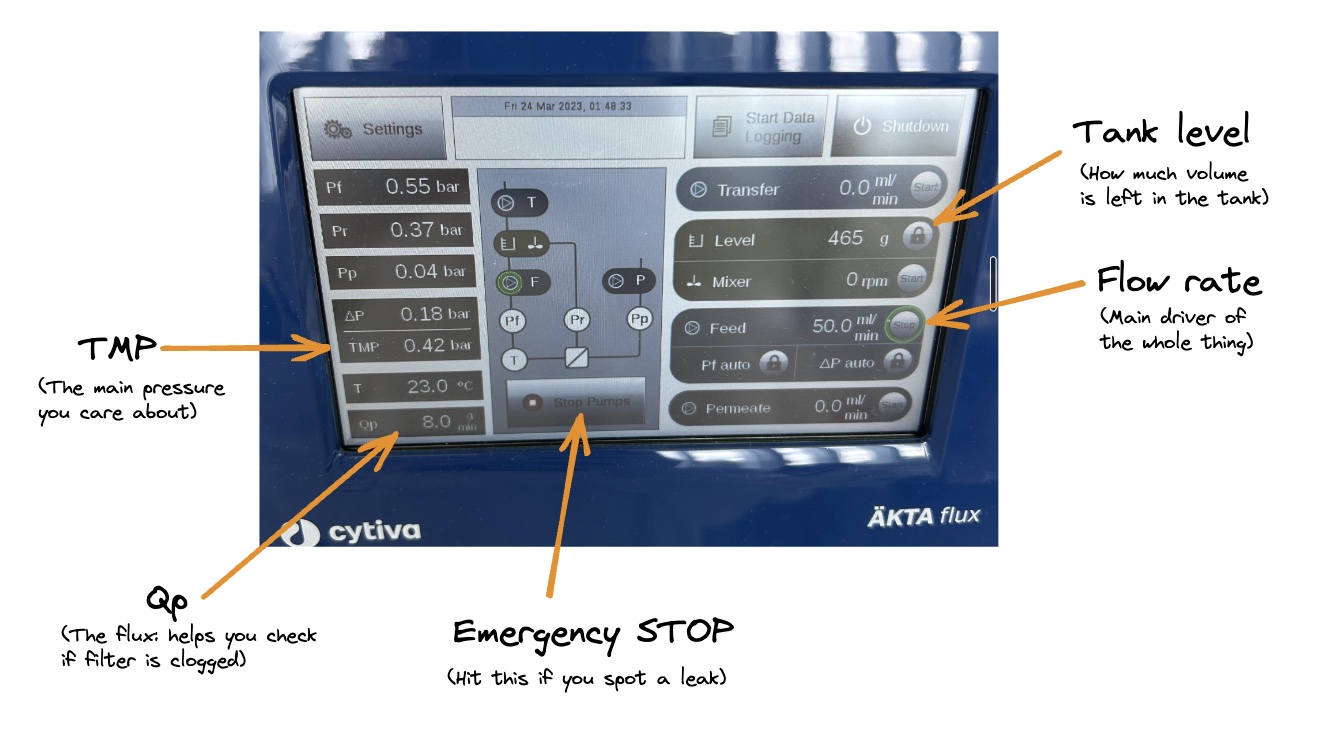
Fig. 4. Here’s a picture of the AKTA Flux S’s screen, and the main things I pay attention to, just to give you an overview. Flow rate: how fast is your pump sending fluid through. You’ll always start with turning this on. TMP: transmembrane pressure — the key thing you manipulate to control how vigorously you’re filtering (you start without any pressure applied, and gradually increase it until you’re at the level you want). Qp: flux (a measure of how much fluid is crossing the membrane and coming off into waste — too low, and you might have a clog).
Monitoring it
Your touch screen tells you what the flow rate is, what the pressure is, what the tank level is, etc. You don’t have to stare at it, but obviously it’s helpful to have.
There is also a place for a USB drive to be inserted, and a button you turn on to ‘Start Logging’. I haven’t played with this yet, but I’m told it records everything automatically so you can maintain an exact record of what was done to your batch.
Should you get one?
If you’re making phages for patients (or even animals or plants), and are trying to do so repeatedly, in a clean, reproducible, demonstrable way, then I think it’s worth considering, and if you’re keen, get a quote from Cytiva and put it into your next grant. Even if you’re making small-scale batches — the AKTA Flux is more about the quality of the process than the scale (it took me a while to realize this). For most phage labs, the model you probably need is either the Flux S (S for Small — this is the one I use). But you may want the Flux 6 (bigger version of the same thing).
What kind of scale can it handle?
I’ve found the Flux S to be able to handle the scale we’re at really well, and I’m not feeling limited by it. I was using 150 mL cultures, then we moved up to 350 mL. The Flux S is perfect for that. It has a tank volume of 500 mL.
Even if we wanted to go higher, to something like 1 L, that would be okay on the Flux S even though it’s higher than the tank volume (super important concept I didn’t realize for a while!). This is because there’s something called a ‘transfer pump’ that lets you auto-transfer liquid from your bottle into the tank (I haven’t played with this yet). But just note that your phage does not all have to fit into the tank at once. (I’m sure there’s an upper limit that would feel practical, but I haven’t looked into that yet).
We haven’t really needed bigger batches than the Flux S can handle yet, since we get a few thousand doses from a few hundred mL of culture, and that’s all we need of any given phage for now. (Case in point, we have both Flux S and Flux 6, but the 6 is still sitting untouched on the bench. It’s got an 8-L tank…!)
Another note about volume — some methods of doing TFF involve a large dilution of the phage before running it (something like 10x dilution in buffer before you even start). If we did that, we’d have 350 mL become 3.5 L… so in that case, I’d probably switch to the Flux 6. I may try this in the future and see how it compares to my current process, but I’ve been told that it’s optional to do the dilution first. Instead, what I do is concentrate first (I take my 350 mL volume down to 50 mL), then top that up a 3-4 times with 3 volumes of buffer, concentrating it back down to 50 mL between each top-up. You end up getting roughly the same dilution overall, but it keeps the volumes smaller. I’m not sure what’s best or what matters yet! For now, as you’ve maybe seen in my past posts, my favourite strategy is still ‘pick something that works and don’t change it until you need to’.
Can it be part of a GMP process?
Yes, the Flux systems can be part of a GMP process (though anything can, in theory). GMP isn’t one thing (that’s another story for another day, which I’m also learning about lately). GMP is more about proving you do your thing in the way you say, rather than being one prescribed thing.
I can already see how something like the Flux would make it much easier to move toward a GMP compliant process, (compared to something like spin columns or CsCl gradients), since it collects data for you about how the run was done, and whether anything went wrong. So if you’re trying to standardize your process, or thinking of one day making your process GMP-compliant, or something close, using something like this is going to make things easier.
Is it chromatography?
A lot of people ask me this. No. The Flux is set up for filtration, but not chromatography. They have the AKTA Pure for that, it’s a similar system of computer+pump+tubes, but it’s set up for doing column chromatography. So you’d connect a column, like say an anion exchange column, or an Endotrap column, instead of a filter.
Excitingly, I get to get trained on our new AKTA Pure next week! So you’ll eventually be hearing about that here too.
Can I reuse the filter? How do I know it’s clean?
I think about two kinds of clean: no more phages in the tubing, and nothing clogging the filter. We need ways of cleaning both, and ways of checking for both things.
To clean out the phages and other lysate components, it’s simple. We’ve been using 0.5 M NaOH, heating it up to 50C, and doing a good amount of flushing/incubating the system with this. To check if it’s clean, we do a titre of the fluid coming through the tubing after cleaning. We are looking for zero phage plaques at the end. If it’s not zero, it’s not clean.
To check if the filter is clogged, there is a handy test called the ‘water flux’ test. You check how fast water comes through the filter (you collect water out the waste tube for 2 mins, check how many mLs you got, and input it into a handy equation that calculates the ‘flux’ (L/m^2/h), where m^2 refers to the surface area of your filter). Then you compare that to how fast it came through last time. If it’s roughly the same, you’re good. If it’s drastically lower, you probably have a clog and should either try cleaning the filter again (maybe with some hot NaOH), or you should replace it. Each is probably good for around 10 runs, or so I’ve heard, but not a lot more than that.
Do the same settings work on lots of phages?
I have tried using the Flux S on 3 phages (all Pseudomonas though) and it’s worked with the same settings, pretty much. My metrics: I haven’t had any phages lost through the filter, and their titres have been high enough to move on to the next purification step. Given this, I think I’ll be using pretty much the same process from here on out on all phages, unless I have a reason to change something.
Does it reduce endotoxin?
So far I’ve seen that it does, to an extent (and Luong et al. show that their TFF system reduces endotoxin quite a lot). But so far in my experience, the TFF process has not been enough on its own to get the endotoxin as low as we need for patients — we still need a second step after, like Endotrap. But TFF does bring EU/mL down to a pretty manageable level, which makes Endotrap work better.
Taking samples
I like to take a lot of samples (a 1-mL aliquot each) during the TFF process, and I titre them after the run to check what all happened. The samples I take:
- Before the run (starting lysate)
- After the first concentration step
- After each wash + concentration step (3-4 more samples)
- Permeate (did I lose any phage?)
- After cleaning (was cleaning sufficient?)
How much does it all cost?
I am not actually sure, but ballpark I believe it’s around 20-30k USD for the Flux S (small one that I use) and around double that for the Flux 6 (bigger one with 8-L tank). Obviously don’t quote me on this… you’ll have to reach out to Cytiva and get a real quote. And I think the filters we use are a couple hundred dollars each (and we’ll be aiming to reuse them around 10x each).
Things I wish it did
- I wish the pressure didn’t drift; this is one thing I find I have to keep an eye on. And it’s the part that’s not digital, so you have to very gently tweak it fairly often.
- I wish the Flux let you upload a fully automated protocol so you didn’t have to stand there at all (the AKTA Pure apparently does this!).
- I wish there was an app so I could monitor it from my phone from the office, or while doing other things. The alarm is sometimes too quiet to hear from very far away.
- I wish it didn’t take so long to clean, so I could run more phages in a day. For now I can only do one in a day (could squeeze in 2 but it’d be a long day). (Soon, when I’m feeling extra confident in my AKTA skills, I will try to run both our machines at once to do two phages at once).
Things I’m still excited to learn
- Using the data collection function — what does the machine collect? Is it helpful/easy enough to process and understand? How might I set up automated ways of visualizing it so I can present this as part of our phage batch reports?
- Playing around with alarms and autofilling when the tank gets to a certain level, so it can become even more hands-off
- What happens if I raise the pressure — can I get rid of more endotoxin? What’s the highest I can comfortably go to without starting to lose phage?
- How much faster can I make the process?
- Is it better to dilute first or do successive washes?
Are there other options for cleaning phage?
Yes. If you’re not too concerned about formally demonstrating the cleanliness, or having fine-tuned control over the process, there are cheaper options for cleaning phages in a similarly hands-off way.
I looked into Sartorius Vivaflow, and I think that’s a pretty good option that you can get for a few thousand (about 5-10x cheaper than AKTA Flux); you just need a peristaltic pump, tubing, and your filter. (Caveat: I haven’t used one of these, have just read about them and talked to Sartorius reps/looked them up online. See Luong et al. for a detailed method using this system for phage cleaning).
As I’ve gathered, the Vivaflow is pretty much the same kind of filter and the same ability to auto-process lots of fluid as the AKTA Flux, but a less sophisticated ‘brain’ telling it all what to do and recording what happens. So this gets around the volume limitation and annoyingly hands-on work of the a spin column. But you won’t have the same control over the process (you’ll report pressure as ‘when you squeeze the tubing during the run, it feels pretty solid to the touch’ instead of ‘0.4 Bar’). You’ll probably have to document the data collection yourself. What flow rate did you use at each step? What was the flux across the filter throughout the process? Did the process stop and start at all? How much volume was processed? You also won’t be able to set it to stop when it gets to a certain volume or pressure. This is probably fine for most phage labs, even ones making phages for patients, but things to consider.
There’s also spin columns (we use the Amicon brand), which are the cheapest/most basic version I know to clean a phage prep. It’s a benchtop centrifuge filter that retains your phage, and lets the liquid and smaller things go through. They work great for very small scales, especially if you’re making a lot of different phages — the one thing AKTA systems (and Vivaflow) don’t do well is many phages in parallel. It’s one phage a day with them… Whereas we’ve done 6 at a time with spin columns, and there’s no cleaning step because you don’t reuse the columns. With spin columns, you’re most limited by the volume you can have at the start. It takes all day to process 150 mL, and the filter starts to clog by the end. Plus, you end up having to concentrate all your phage into a tiny volume, and some phages don’t like that.
Parting thoughts
I hope I’ve made the case that the AKTA Flux is useful, and is simple enough for any phage biologist to use! You can get it up and running pretty easily, and it will save labour time and make whoever is responsible for phage-making less stressed, and feel less like a manual labourer and more like a proficient robot operator (you may even have them wanting to make phages all the time).
And if your team wants to transition to a GMP-compliant process, or even ‘GMP-light’, or even just wants to reassure your regulator or local hospital committee that you are doing things cleanly, and progressing toward a system that can be done reproducibly, predictably, and which can one day be audited, this can be a great step up.
Coming up next
After TFF, I have been doing Endotrap purification. I’ll be exploring AKTA Pure for doing this step soon, and I’ll be blogging about this too. I may also do a post on the Endotrap columns, as you can also run them without an AKTA attached to them, which we’ve been doing, and it’s been working pretty well!
Thanks for reading! See you next time!
— Jessica
Further reading
Cytiva AKTA Flux website
Sartorius Vivaflow website
Great paper, detailed method on using Sartorius system for phage purification:
Luong, T., Salabarria, A. C., Edwards, R. A., & Roach, D. R. (2020). Standardized bacteriophage purification for personalized phage therapy. Nature Protocols, 1-24.











